Epstein-Barr virus (EBV), or the human herpesvirus 4 (HHV-4), is one of the eight known human herpesviruses of the gamma herpes virus family and is the primary causative agent of Infectious mononucleosis (IM).
It was first discovered in cells isolated from African Burkitt’s lymphoma and has also been associated with nasopharyngeal carcinoma (NPC), gastric carcinoma (GC), Hodgkin’s syndrome, some T-cell lymphomas, and post-transplant lymphoproliferative disease (PTLD).
Interesting Science Videos
Structure of Epstein-Barr Virus (EBV)
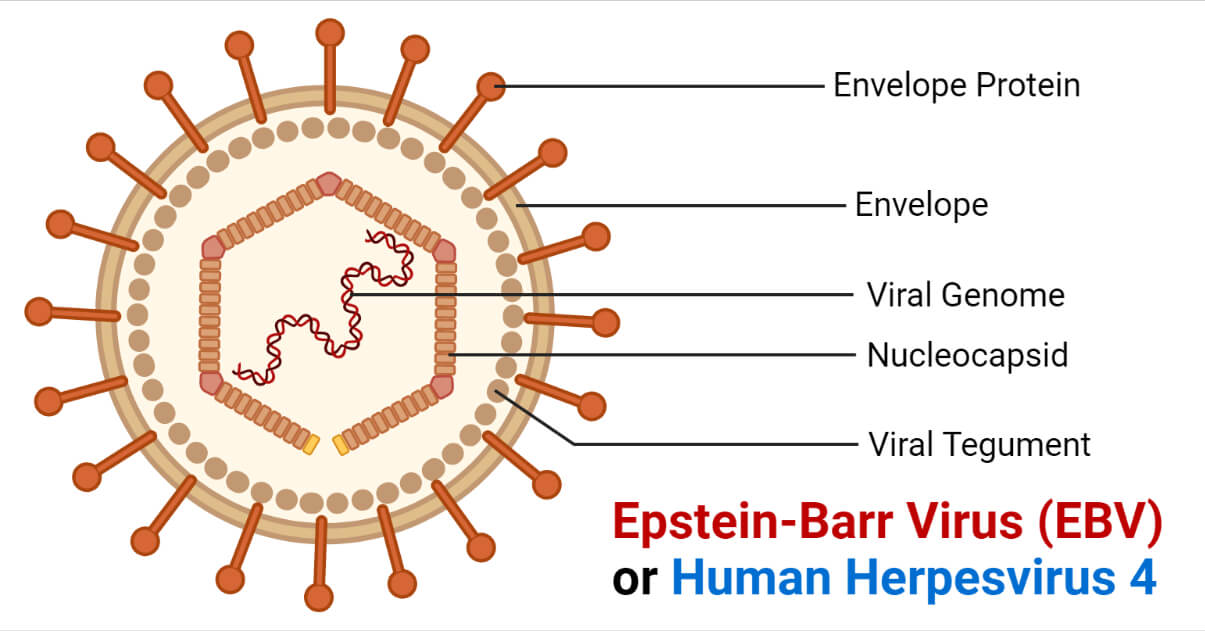
- The structure of EBV is similar to other herpesviruses.
- It consists of a double-stranded DNA surrounded by an icosahedral protein capsid containing 162 capsomers.
- A protein tegument is present between the capsid and the envelope embedded with glycoproteins that play a part in cell tropism, host range, and cell recognition.
- The mature virions are approximately 120 to 180mm in diameter.
- There are currently two recognized subtypes of EBV; Type 1 and Type 2, also referred to as Type A and Type B, respectively.
- These subtypes differ from one another at the EBV nuclear antigen loci (EBNA).
Genome structure of Epstein-Barr Virus (EBV)
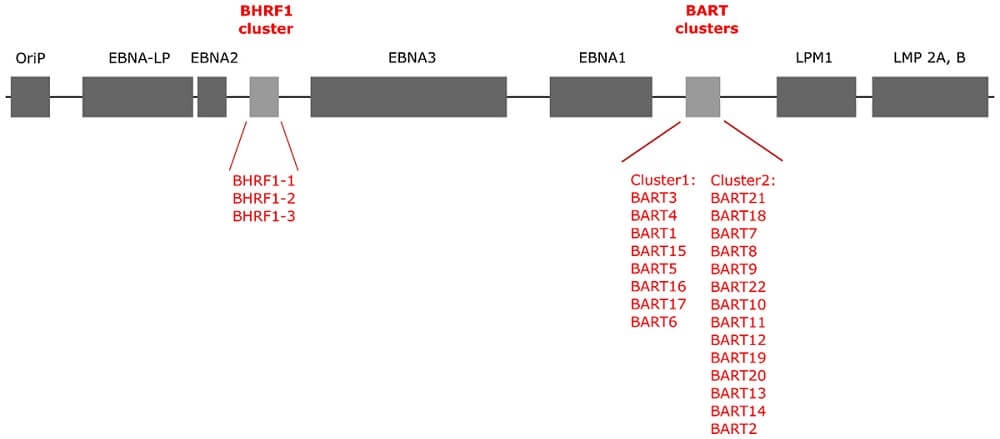
- The genomic structure of the EBV virus consists of a linear, double-stranded DNA of approximately 172 Kbp genome size, which encodes for more than 85 genes.
- The genome consists of uniquely tandem repeats of DNA fragments, whose number varies among different EBV isolates.
- Most proteins encoded by the virus are involved in the virus metabolism, replication cycles, and building the structural compartments like nucleocapsid, tegument proteins, and the envelope.
- The EBV genome also consists of several latent genes that remain untranslated during the lytic phase and several RNA genes that are expressed during latency.
- During latency, the EBV DNA usually persists as multiple circular episomes inside the infected cells. However, it can also integrate with the chromosomal DNA and persist as integrated DNA.
Epidemiology of Epstein-Barr Virus (EBV)
- The lymphotropic virus EBV is a widespread and highly prevalent virus among a wide range of populations.
- It is estimated to be positive in more than 90% of the world’s population.
- The primary infection is generally asymptomatic, occurring in childhood. The occurrence of the virus is observed to be influenced by socioeconomic factors like poor hygiene and sanitation, overcrowding, etc.
- In developed countries with better hygiene conditions, the EBV seroconversion was observed to be the highest in children between 2 to 4 years and also in 14 to 18 years, increasing with the increase in age.
- However, in developing countries with poor hygiene conditions, it is usually acquired in early childhood, with almost all children testing seropositive by the age of 6 years.
Transmission of Epstein-Barr Virus (EBV)
- The oral route is the primary route of transmission for the EBV virus through saliva containing the infected cells.
- Activities like kissing, sharing personal belongings like toothbrushes, eating utensils, and sharing food and drinks can also aid in virus transmission.
- It can also spread through blood transfusions and organ transplantations.
- Although blood banks undergo numerous screening procedures for infectious pathogens, the risk of transmission of untested pathogens, such as HEV, CMV, and EBV, still remains of concern.
- The presence of infected cells in the uterine cervix and semen is also suggestive of transmission through the sexual route.
Replication of Epstein-Barr Virus (EBV)
1. Attachment/Adsorption
After the entry of the virus through any of the transmission routes, the B cells and epithelial cells act as host cells for the virus. The virus attaches through receptors like CD21 and integrin proteins on the host cells by interaction of the receptors with the viral glycoproteins.
2. Penetration
The EBV virus penetrates into the host cells through the process of fusion. The viral protein gp42 interacts with the HLA class II molecule of the B cell, while the EBV gH/gL envelope protein interacts with the αvβ6/8 integrins of the epithelial cells that induce the fusion with the host cell and allow the entry of the viral particle.
3. Uncoating
Through the action of host lysosomal enzymes, the capsid gets separated from the viral genome, and the viral DNA is released into the cytoplasm that enters the nucleus.
4. Biosynthesis
The linear dsDNA of the EBV gets converted to circular DNA and is replicated through the rolling circle mechanism. During the viral latency, latent genes are transcribed, and the circular DNA can persist for decades before entering the lytic phase. In the lytic phase, the intermediate-early, early, and late mRNA are synthesized that leave the nucleus and get translated to proteins in the free ribosomes or ribosomes on the endoplasmic reticulum.
5. Assembly
The capsid proteins enter the nucleus and form the nucleocapsid with the viral genome, and bud off the nuclear membrane with a single membrane envelope.
6. Maturation
The maturation of the virus takes place in the endoplasmic reticulum and Golgi bodies and gets released back to the cytoplasm.
7. Release
The viruses are released from the infected host cells through the lysis of the host cell membrane.
Pathogenesis of Epstein-Barr Virus (EBV)
The pathogenesis of the EBV can be divided into three phases:
1. Primary Infection and Lytic Replication
- The primary infection usually begins in the tonsillar region, with the virus mainly affecting the lymphocytes and epithelial cells. The EBV binds to CD21 receptors on the B cells through the viral gp350, followed by interaction of viral gp42 with HLA class II molecules of the B cells that induce fusion with the host membrane.
- In epithelial cells, EBV BMRF-2 protein interacts with the β1 integrins, followed by the interaction of EBV gH/gL envelope protein with αvβ6/8 integrins initiating their fusion.
- The viral replication, transcription, and translation produce a range of gene products where the early products are involved in many functions, including replication, metabolism, and suppression of antigen processing, while the late gene products are involved in the production of structural proteins and products involved in immune evasion.
- The infected B cells are triggered to differentiate into memory B cells and released into peripheral circulation resulting in high virus levels in the blood. Although the number of infected B cells decreases with time after the onset of symptoms from primary infection, they are never completely eliminated.
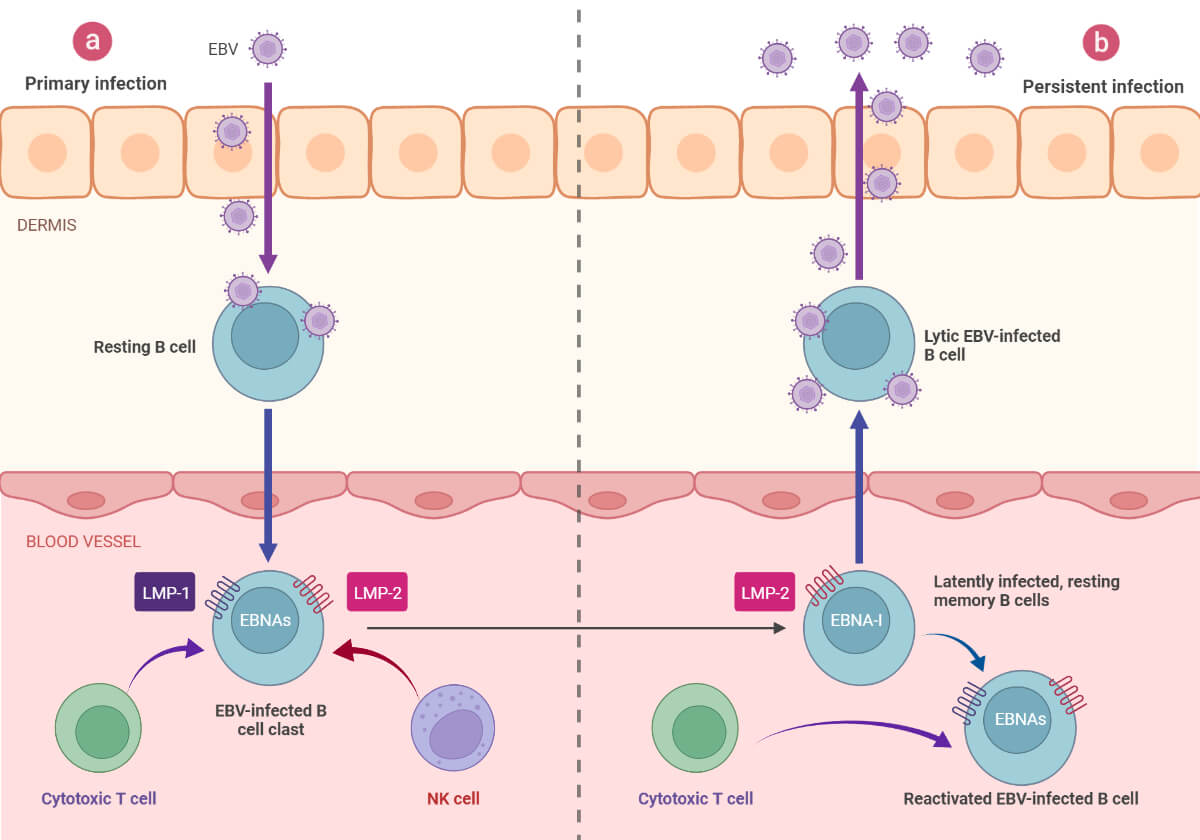
2. Latency
- The EBV genome mostly persists in the B cells as episomes or as integrated DNA and may also exist in the epithelial cells.
- During the latent phase, replication occurs through the host DNA polymerase, and limited EBNA (EBV nuclear antigen) and LMP (latent membrane proteins) are expressed.
- The latency phase occurs as three different latency programs.
- Through transcription, the latent EBV gene can multiply in memory cells, induce B cell differentiation, activate the naïve B cells, or even restrict the expression of all genes.
- During type I latency, only EBNA1 is expressed, as observed in Burkitt’s lymphoma. CD8 T cells are produced in response to specific EBV antigens but not against EBNA1, which helps them evade the host immune response.
- During type II latency, EBNA1 and LMP1/2A are expressed as seen in nasopharyngeal carcinoma and Hodgkin’s lymphoma.
- All the latency gene products are produced during the type III latency observed during acute infectious mononucleosis complication where the infected B cells become immortalized, and a large number variety of antibodies are produced against it.
3. Reactivation
- The exact cause for the viral reactivation is not yet clear however chronic psychological stress and the weakened immune system of the host have been observed to induce the reactivation.
- The latently infected cells further infect new B cells and epithelial cells that aid in the viral shedding and transmission.
- The proportion of EBV-infected cells that are in the lytic or latent phase at any given time is, however, not known.
Clinical Manifestations of Epstein-Barr Virus (EBV)
Many people get infected with EBV during their early childhood. However, they may be asymptomatic or have generalized symptoms similar to other common childhood diseases.
However, in rare cases, the infection can lead to serious consequences.
The symptoms of EBV can include:
- Fever
- Fatigue
- Inflammation of throat
- Swollen lymph nodes in the neck
- Enlarged spleen
- Swollen liver
- Rash
Complications of the EBV include:
- Infectious mononucleosis
- Cervical lymphadenopathy with lymphocytosis
- Palatal petechiae
- Hepatitis
- Splenic rupture
- Erythroblastopenia
- Thrombocytopenia
- Neurological disorders (meningoencephalitis, Guillain-Barré syndrome, Bell’s palsy)
Late complications of EBV can also include:
- Lymphoproliferative cancers
- Multiple sclerosis
- Rheumatoid arthritis
- Chronic active Epstein-Barr virus infection (CAEBV)
Diagnosis of Epstein-Barr Virus (EBV)
Non-Specific Tests
Peripheral blood smear
The presence of atypical lymphocytes is seen in the peripheral blood of patients with infectious mononucleosis. These cells, also known as Downey cells, are activated CD8 T cells produced as a response to the EBV-infected B cells.
Heterophile antibody test
It is considered the best initial diagnostic test for EBV-related IM. About 85% of patients with EBV-related IM will test positive for the test. However, the probability of a false negative result for 25% of patients is possible during the first week of symptoms due to lower antibody titers.
Liver function test
About 80% of patients with infectious mononucleosis show abnormal liver function tests during the early stages of infection with increased levels of enzymes, especially alanine aminotransferase.
Specific Tests
EBV-specific antibody test
EBV viral capsid antigen (VCA) IgG and IgM antibody tests are used in the diagnosis during the acute phase of the disease.
EBNA antibody test also helps to differentiate between acute and past infections.
VCA and EBNA antibody tests have higher sensitivity and specificity however, they are relatively expensive and more time-consuming.
Viral detection and quantification
EBV-encoded RNA transcripts (EBERs) is considered the gold standard for detecting EBV in tissues.
The virus can also be detected and quantified through molecular methods like PCR.
Treatment of Epstein-Barr Virus (EBV)
- There is no specific treatment for the EBV virus infection. However, symptomatic management of infectious mononucleosis can be done by the use of antipyretics, and analgesics to reduce fever and pain during the acute phases.
- Oral rehydration and a nutritional diet can be especially important for febrile patients.
- Proper bed rest, use of corticosteroids, and antiviral drugs like acyclovir and valacyclovir can also be used in the treatment of the infection.
Prevention and Control of Epstein-Barr Virus (EBV)
It is rather difficult to prevent the infection of the Epstein-Barr virus. There is no vaccine that is developed specifically to protect against EBV infection. Some preventive measures that can be adopted include:
- Avoiding activities like kissing, sharing foods, drinks, personal belongings like toothbrushes, eating utensils, etc. with people infected with EBV
- Avoiding sharing of toys that may contain drools and saliva of infants
- Washing hands and maintenance of proper personal hygiene helps mitigate the transmission of the virus
- Proper screening and testing of blood in the blood banks for the viruses can also reduce the risk of transmission of the virus
References
- Odumade, O. A., Hogquist, K. A., & Balfour, H. H. (2011). Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clinical Microbiology Reviews, 24(1), 193–209. https://doi.org/10.1128/cmr.00044-10
- Fugl, A., & Andersen, C. L. (2019). Epstein-Barr virus and its association with disease – a review of relevance to general practice. BMC Family Practice, 20(1). https://doi.org/10.1186/s12875-019-0954-3
- Apurba Sankar Sastry, & Sandhya Bhat K. (2019). Essentials of medical microbiology. Jaypee Brothers Medical Publishers.
- Okano, M., Thiele, G. M., Davis, J. R., Grierson, H. L., & Purtilo, D. T. (1988). Epstein-Barr virus and human diseases: recent advances in diagnosis. Clinical Microbiology Reviews, 1(3), 300–312. https://doi.org/10.1128/cmr.1.3.300
- Smatti, M. K., Al-Sadeq, D. W., Ali, N. H., Pintus, G., Abou-Saleh, H., & Nasrallah, G. K. (2018). Epstein–Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Frontiers in Oncology, 8. https://doi.org/10.3389/fonc.2018.00211
- Thorley-Lawson, D. A., Hawkins, J. B., Tracy, S. I., & Shapiro, M. (2013). The pathogenesis of Epstein–Barr virus persistent infection. Current Opinion in Virology, 3(3), 227–232. https://doi.org/10.1016/j.coviro.2013.04.005
- Frappier, L. (2021). Epstein-Barr virus: Current questions and challenges. Tumour Virus Research, 12, 200218. https://doi.org/10.1016/j.tvr.2021.200218
- Epstein-barr (EPV). (2019). CDC. https://www.cdc.gov/epstein-barr/index.html
- Young, L. S., Arrand, J. R., & Murray, P. G. (2007). EBV gene expression and regulation (A. Arvin, G. Campadelli-Fiume, E. Mocarski, P. S. Moore, B. Roizman, R. Whitley, & K. Yamanishi, Eds.). PubMed; Cambridge University Press. https://www.ncbi.nlm.nih.gov/books/NBK47431/#:~:text=The%20EBV%20genome%20is%20composed
- Smatti, M. K., Al-Sadeq, D. W., Ali, N. H., Pintus, G., Abou-Saleh, H., & Nasrallah, G. K. (2018). Epstein–Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Frontiers in Oncology, 8. https://doi.org/10.3389/fonc.2018.00211
- Kerr, J. R. (2019). Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. Journal of Clinical Pathology, 72(10), 651–658. https://doi.org/10.1136/jclinpath-2019-205822

please get me your pdf