- Enzymes are the biological macromolecules, also called as biological catalysts, which speed up the rate of biochemical reactions without undergoing any change.
- It is a highly selective catalyst that greatly accelerates both the rate and specificity of metabolic reactions.
- Many types of molecule exist which are capable of interfering with the activity of an individual enzyme.
- Any molecule which acts directly on an enzyme to lower its catalytic rate is called as an inhibitor.
- Some enzyme inhibitors are normal body metabolites that inhibit a particular enzyme while other inhibitors may be foreign substances, such as drugs or toxins.
- The inhibition may be a part of the normal metabolic control of a pathway, a diseased condition or either a therapeutic measure.
- Thus, the effect of enzyme inhibition could be either therapeutic or, at the other extreme, lethal.
- Enzyme inhibition may be of two main types:
- Irreversible Inhibition
- Reversible Inhibition
- Reversible inhibition may be further subdivided into:
- Competitive reversible inhibition and
- Non-competitive reversible inhibition.
Interesting Science Videos
1. Irreversible Inhibition
- Irreversible inhibitors bind tightly to the enzyme and inactivate it.
- Inhibitors which bind irreversibly to an enzyme often form a covalent bond to an amino acid residue at or near the active site, and permanently inactivate the enzyme.
- Susceptible amino acid residues include Ser and Cys residues which have reactive –OH and –SH groups, respectively.
Examples
- The compound di-isopropylphosphofluoridate (DIPF), a component of nerve gases, reacts with a Ser residue in the active site of the enzyme acetylcholinesterase, irreversibly inhibiting the enzyme and preventing the transmission of nerve impulses.
- Iodoacetamide modifies Cys residues and hence may be used as a diagnostic tool in determining whether one or more Cys residues are required for enzyme activity.
- The antibiotic penicillin irreversibly inhibits the glycopeptide transpeptidase enzyme that forms the cross-links in the bacterial cell wall by covalently attaching to a Ser residue in the active site of the enzyme.
- A nonspecific inhibitor affects all enzymes in the same way. Non-specific methods of inhibition include any physical or chemical changes which ultimately denature the protein portion of the enzyme and are therefore irreversible.
2. Reversible Inhibition
- Reversible inhibition can be overcome by removing the inhibitor from the enzyme.
- Reversible enzyme inhibitors can be classified as either competitive or noncompetitive, and can be distinguished via a Lineweaver–Burk plot.
Competitive Inhibitors
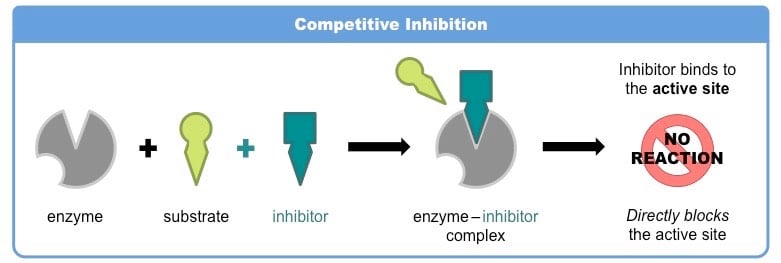
- Competitive inhibitors compete with the substrate for the active site of the enzyme and form an enzyme–substrate complex.
- A competitive inhibitor typically has close structural similarities to the normal substrate for the enzyme. Thus it competes with substrate molecules to bind to the active site.
- Since, the enzyme may bind either a substrate molecule or an inhibitor molecule, but not both at the same time; binding to the inhibitor inhibits its activity.
- The competitive inhibitor binds reversibly to the active site.
- At high substrate concentrations the action of a competitive inhibitor is overcome because a sufficiently high substrate concentration will successfully compete out the inhibitor molecule in binding to the active site.
- Many drugs work by mimicking the structure of the substrate of a target enzyme, and hence act as competitive inhibitors of the enzyme.
Example
A good example of competitive inhibition is provided by succinate dehydrogenase. The enzyme uses succinate as its substrate and is competitively inhibited by malonate which differs from succinate in having one rather than two methylene groups
Non- competitive Inhibitors
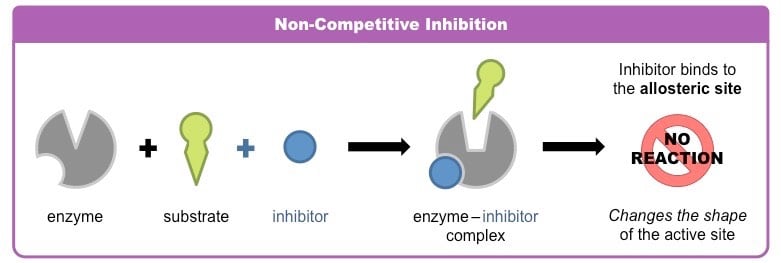
- Non- competitive inhibitors bind to the enzyme or the enzyme–substrate complex at a site different from the active site, decreasing the activity of the enzyme.
- A noncompetitive inhibitor binds reversibly at a site other than the active site and causes a change in the overall three-dimensional shape of the enzyme that leads to a decrease in catalytic activity.
- Since the inhibitor binds at a different site from the substrate, the enzyme may bind the inhibitor, the substrate or both the inhibitor and substrate together.
- The effects of a non-competitive inhibitor cannot be overcome by increasing the substrate concentration, so there is a decrease in Vmax.
- An example of noncompetitive inhibition is the action of pepstatin on the enzyme renin.
References
- Suzanne J. Baron and Christoph I. Lee (2013).Biochemistry & Genetics. Second Edition. Mc Graw Hill: New York.
- David Hames and Nigel Hooper (2005). Biochemistry. Third ed. Taylor & Francis Group: New York.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
