Glycolysis is the sequence of catabolic reactions converting glucose (or glycogen) molecule to pyruvic acid using several enzymes. In the process, a glucose molecule is catalyzed to yield two molecules of pyruvic acids, two molecules of NADH, and two molecules of ATP. It is also called “Embden-Meyerhof-Parnas Pathway” or “EMP Pathway”.
It is the first stage of cellular respiration occurring in the cytoplasm of every cell. It occurs in both aerobic and anaerobic organisms. It is a complex 10 step catabolic reaction that enzymatically catalyzes a glucose molecule into two 3-carbon compounds; either pyruvic acid (pyruvate) or lactic acid (lactate). In aerobic respiration, pyruvate is the final product, whereas lactate is the final product in anaerobic respiration. It is the major pathway for ATP generation in cells lacking mitochondria.
The overall reaction of glycolysis can be summarized as:
Glucose + 2NAD+ +2ADP + 2Pi → 2Pyruvate + 2NADH + 2ATP + 2H2O + 2H+
Interesting Science Videos
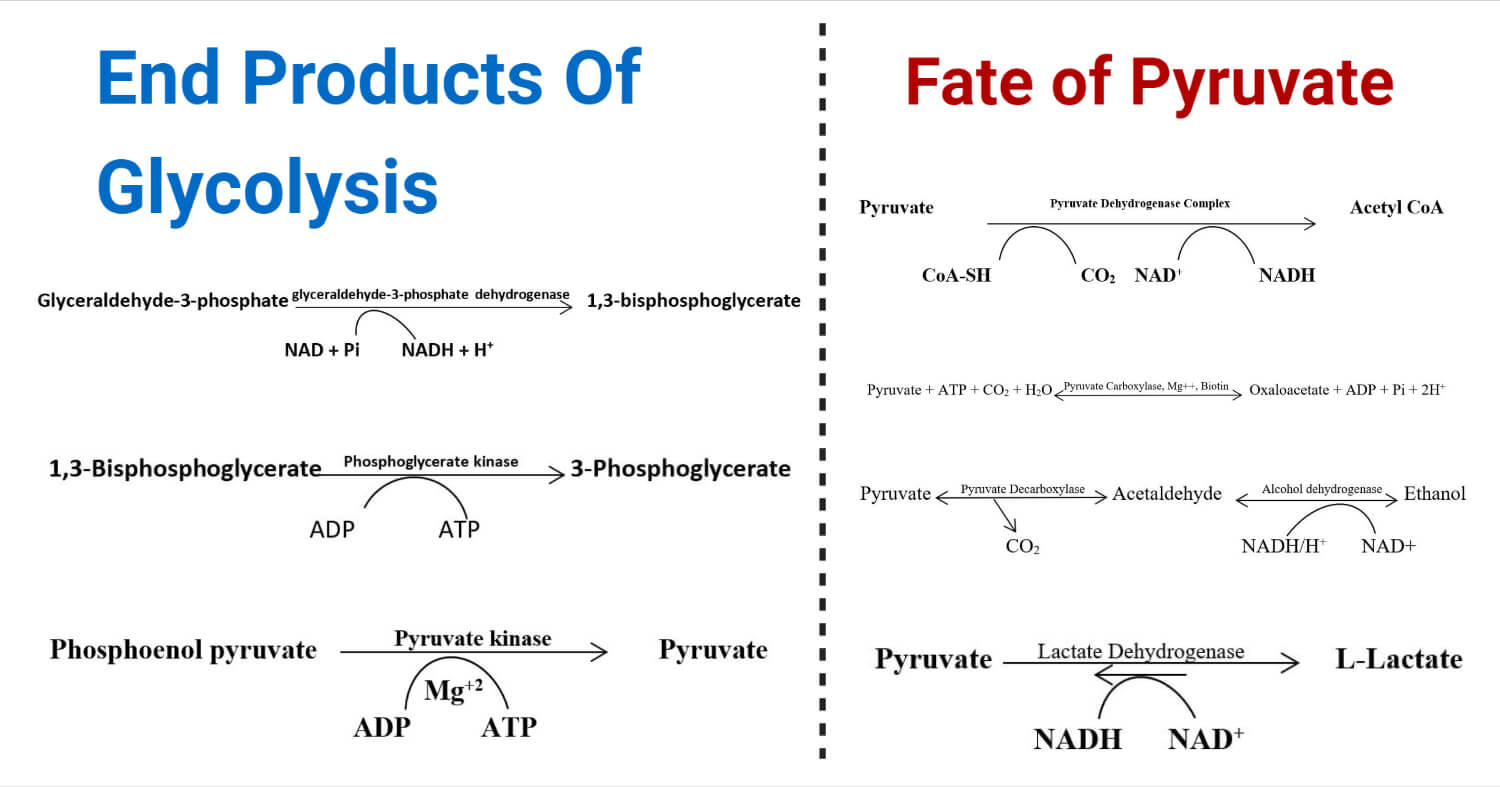
Products of Glycolysis
During a glycolytic pathway, a glucose molecule is enzymatically degraded, producing either 2 pyruvates (in aerobic type) or 2 lactate (in anaerobic type) as the major product, 2 ATP (adenosine triphosphate) molecules, 2 NADH (Nicotinamide adenine dinucleotide), 2 water molecules, and 2 protons (H+).
1. ATP (Adenosine Triphosphate)
There are certain organic compounds in biological systems which on hydrolysis yield at least 7 kCal/mol of energy. These compounds are called high-energy compounds. It includes pyrophosphates, acyl phosphates, enol phosphates, thioesters, and phosphagens. ATP is a pyrophosphate molecule that provides energy for conducting metabolic processes i.e. sustaining the life of a cell.
ATP is a complex organic high-energy compound that provides energy for conducting metabolic processes. It is referred to as “the molecular unit of currency” of the intracellular energy transfer or “Energy Currency of the Cell”. It is the source of energy for use and storage.
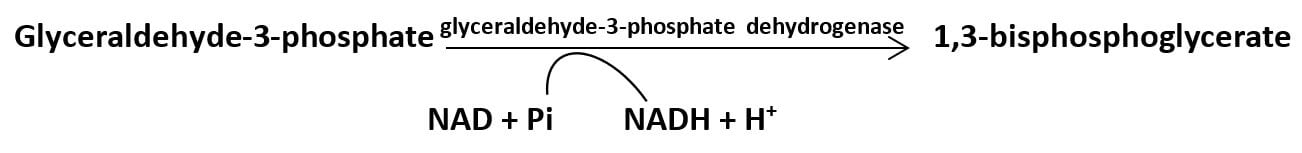
In the glycolytic pathway, oxidation of G-3-P by G-3-P dehydrogenase enzyme adds a high energy phosphate group which is transferred to ADP in the next reaction generating ATP molecule.
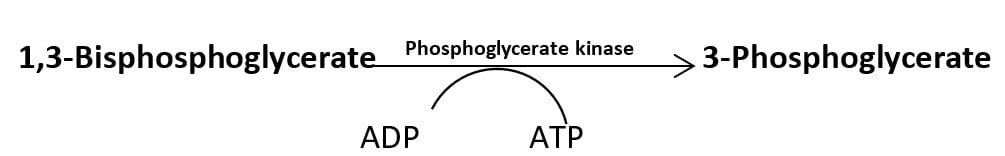
In another reaction, the energy released during dehydration of 2-phosphoglycerate converts the low energy phosphate bond into a high energy phosphate bond which is transferred to ADP in the next reaction producing an ATP molecule.

During cellular respiration, catabolism of organic compounds like polysaccharides, fats, and proteins, a lot of energy is released. This released energy is coupled in the form of ATP inside the cell. The energy is stored in the triphosphate moiety of the ATP. The ATP is hydrolyzed to ADP (adenosine diphosphate) and Pi (inorganic phosphate) resulting in the release of 7.3 kCal/mol energy. The ADP further hydrolyzes into AMP (adenosine monophosphate) and Pi releasing 6.6 kCal/mol of energy.
Chemical formula: C10H16N5O13P3
Molar mass: 507.18 g/mol
Solubility: water-soluble
ATP Structure
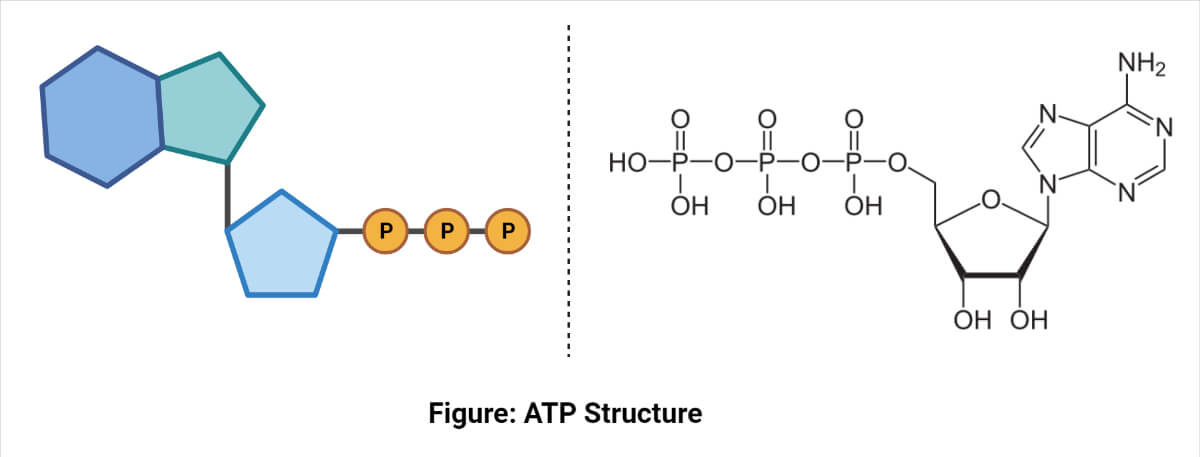
It consists of adenine, ribose, and a triphosphate moiety. Adenosine is attached by the 9-nitrogen atom to the 1-carbon atom of ribose which in turn is attached at the 5-carbon atom of sugar to a triphosphate group.
It plays a major role in anabolic reactions by providing energy. Vital processes like active transport, muscle contraction, impulse transmission, DNA, RNA, protein biosynthesis, cell division, etc. require ATP. It also play role in signal transmission by serving as a substrate for kinases, extracellular signaling, and neurotransmission.
2. NADH (nicotinamide adenine dinucleotide (NAD) + hydrogen (H))
It is the reduced form of coenzyme NAD+ (Nicotinamide adenine dinucleotide). Vitamin B3 (niacin) is the base for the production of NAD+ and NADH. It is stated as “Nicotinamide adenine dinucleotide + hydrogen.” It is also called “coenzyme 1”. It is an essential coenzyme that facilitates numerous cellular biochemical reactions and energy production.
Two molecules of NAD+ are reduced, producing 2 NADH during oxidation of glyceraldehyde – 3 – phosphate to 1,3 – bisphosphoglycerate by the enzyme ‘glyceraldehyde – 3 – phosphate dehydrogenase’.

Thus produced NADH is used as a substrate in the electron transport chain. From one NADH molecule, 2.5 ATP molecules are generated.
Chemical formula: C21H28N7O14P2
Molecular mass: 664.4 g/mol
Solubility: water-soluble
NADH Structure
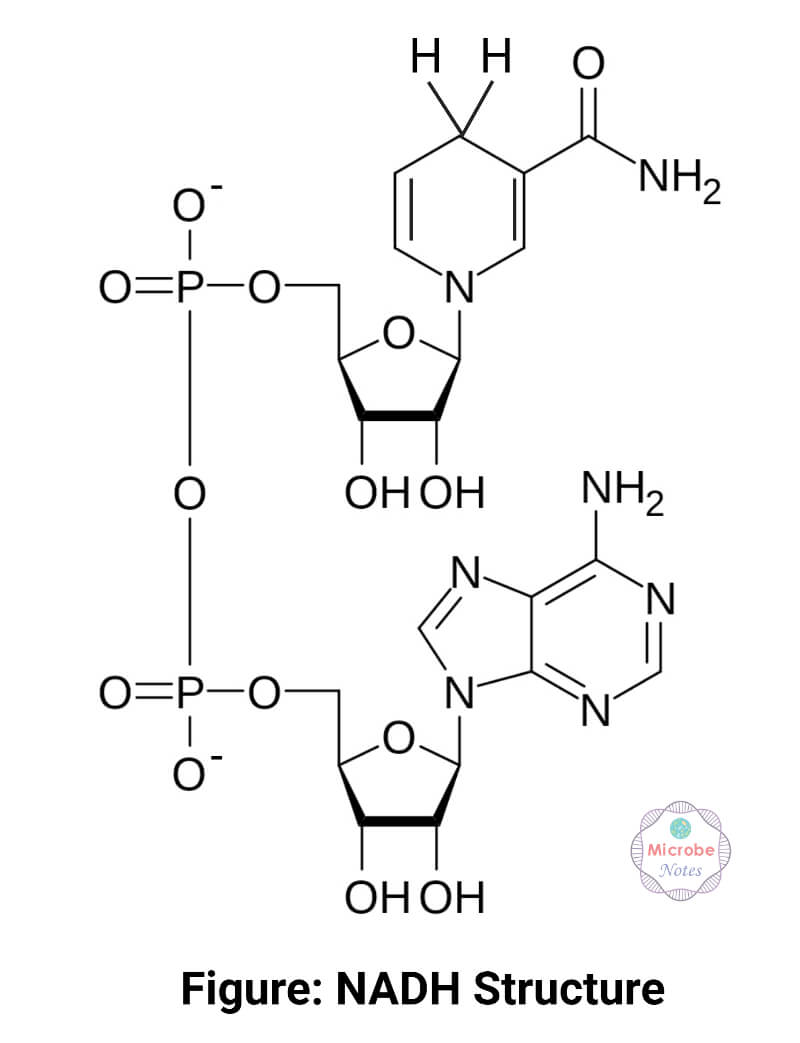
It is made up of 2 nucleosides connected via pyrophosphate linkage. Each nucleoside contains a ribose sugar with a phosphate group at its fifth carbon atom. C1 of the ribose in one nucleoside is attached to the adenine molecule, while the C1 of the ribose in the other nucleoside is attached to the nicotinamide molecule.
It plays a significant role in several metabolic reactions by acting as a coenzyme in several redox reactions. It acts as an electron donor in the cellular respiration process (ETC). It is a substrate for the formation of cyclic ADP ribose, bacterial DNA ligases, sirtuins, NADPH, and many other enzymes. It also plays an essential role in cell signaling processes, cellular communication processes, and neurotransmission.
3. Pyruvate (Pyruvic acid)
Pyruvate is a conjugate base of pyruvic acid formed by deprotonation of the carboxy group of a pyruvic acid molecule. It is a 2-oxo-monocarboxylic acid anion.
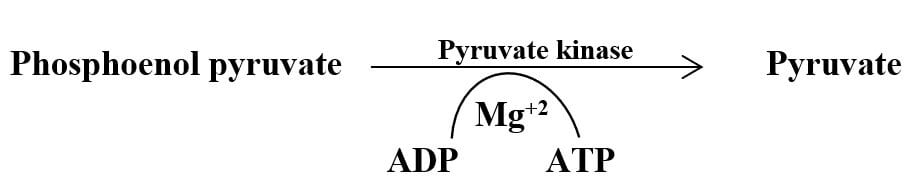
It is the end product of glycolysis. It is produced by the final transfer of a phosphate group from the phosphoenolpyruvate molecule to an ADP molecule in the reaction catalyzed by the enzyme “pyruvate kinase”. Two molecules of pyruvate are produced from a single glucose molecule during glycolysis.
Chemical formula: C3H3O3–
Molecular mass: 87.05 g/mol
Solubility: water-soluble
Pyruvate Structure
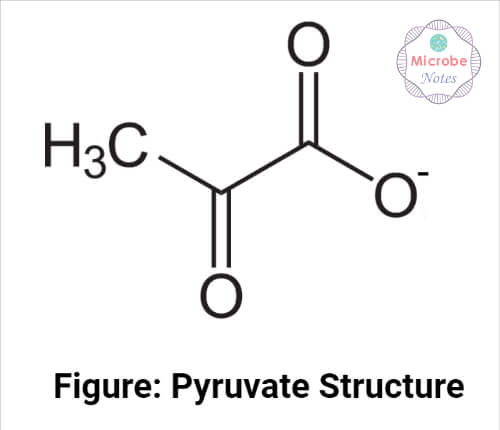
It is a three-carbon molecule with a carboxylic group and ketone as a functional group. C1 forms the carboxylic group. Ketone is attached to the C2, and the C3 contains a methyl group.
It is the carbon source that is transferred to mitochondria for the Krebs cycle and ultimately to ETC for ATP production. It is the substrate for gluconeogenesis, and also in the generation of several non-essential amino acids.
Fate of Pyruvate
Glycolysis produces two molecules of ‘pyruvate’ from a single glucose molecule. These pyruvates can enter into different metabolic reactions and produces energy through different pathways. Based on the pathway they enter, pyruvate may be converted to ethanol, lactic acid, acetyl CoA, or oxaloacetate. Acetyl CoA and oxaloacetate are produced in aerobic respiration, while ethanol and lactate are produced during anaerobic respiration.
A. In Presence of Oxygen
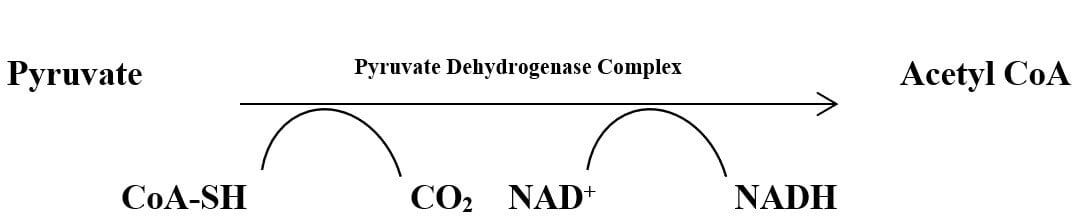
1. Acetyl CoA Formation
Pyruvate can’t enter the TCA cycle, so in the aerobic condition, it is converted to the alternative compound ‘Acetyl Co-A’ which can then enter the TCA cycle. Pyruvate is converted to Acetyl-CoA in the matrix of mitochondria by the enzyme “pyruvate dehydrogenase complex (E1, E2, and E3)” in presence of 5 coenzymes; TPP (Thymine Pyrophosphate), FAD (Flavin Adenine Dinucleotide), Lipoate, CoA-SH, and NAD+. E1(pyruvate dehydrogenase) and TPP complex cause decarboxylation resulting loss of carbon atoms from pyruvate to form CO2. The acetyl group is transferred to CoA-SH by TPP, E2 (dihydrolipoyl transacetylase), and Lipoate complex resulting in “Acetyl CoA” formation. E3 (dihydrolipoyl dehydrogenase) transfers H+ from lipoate to FAD which transfers an electron to NAD+ making NADH + H+.
2. Oxaloacetate Formation
In aerobic conditions, pyruvate may also be converted to oxaloacetate, which can also enter the TCA cycle to generate energy. This carboxylation reaction is catalyzed by the enzyme “pyruvate carboxylase”. This reaction is dependent on the presence of Biotin and Mg+2 ions. Oxaloacetate formation is a very important process to replenish the intermediates of the TCA cycle and the beginning of gluconeogenesis. A molecule of ATP is utilized in the reaction.
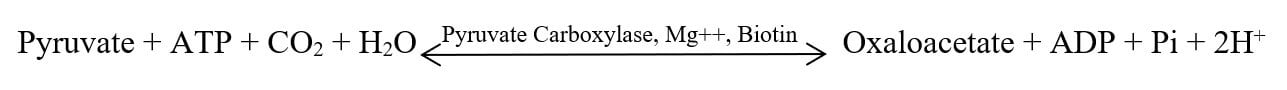
B. In the Absence of Oxygen
1. Ethanol Formation
Ethanol fermentation is another very important process occurring in several yeasts and several other microorganisms. It is a two-step fermentation reaction where pyruvate is reduced to ethanol molecules.
In the first step, pyruvate is decarboxylated into acetaldehyde by the enzyme “pyruvate decarboxylase” in presence of coenzyme TPP and Mg+2 ion. In the second step, the acetaldehyde is reduced to ethanol by the action of the enzyme “alcohol dehydrogenase”. A molecule of CO2 is released in the first step, and a molecule of NADH is oxidized to NAD+ in the second step of the fermentation reaction.

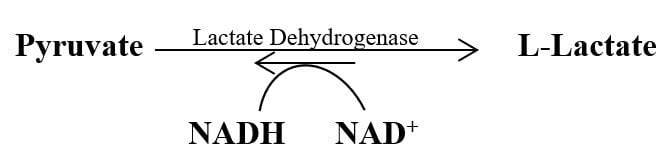
2. Lactate Formation
Pyruvate is reduced to lactate in anaerobic conditions like in skeletal muscles during constant contraction and relaxation, brain cells, retina, RBCs, microbial cells, etc. stretching.
Lactate fermentation reaction is catalyzed by the enzyme “lactate dehydrogenase”. During this process, NADH is oxidized to NAD+, which facilitates the reduction of pyruvate to lactate.
References
- National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 5893, Nicotinamide-Adenine-Dinucleotide. Retrieved March 29, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/Nicotinamide-Adenine-Dinucleotide.
- Melkonian, E. A., & Schury, M. P. (2021). Biochemistry, Anaerobic Glycolysis. In StatPearls. StatPearls Publishing.
- Nelson, D. L., & Cox, M. M. (2017). Lehninger principles of biochemistry (7th ed.). W.H. Freeman.
- Satyanarayana, U. (2013). Biochemistry. Elsevier Health Sciences. https://books.google.com.np/books?id=Bd9XAwAAQBAJ
- ATP – Energy Currency of the Cell – Structure and its Functions (byjus.com)
- Adenosine Triphosphate (ATP) – Definition, Structure and Function (biologydictionary.net)
- Beis I, Newsholme EA. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975 Oct;152(1):23-32.
- Dunn J, Grider MH. Physiology, Adenosine Triphosphate. [Updated 2022 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553175/
- Ziegler M (2000). “New functions of a long-known molecule. Emerging roles of NAD in cellular signaling”. Eur. J. Biochem. 267 (6): 1550–64. doi:10.1046/j.1432-1327.2000.01187.x. PMID 10712584
- what is nadh in biology – Lisbdnet.com
- What is NADH? – Co-E1
- A Glimpse at the Function of NADH and FADH2 in Cellular Respiration – Biology Wise
- Pyruvate – Definition, Structure and Function | Biology Dictionary
- National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 107735, Pyruvate. Retrieved March 29, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/Pyruvate.
- Fate of Pyruvate: Acetyl CoA, Lactate, Alcohol Formation. (microbiologynote.com)
- Fate of Pyruvate (Fate of End product of Glycolytic pathway) (microbenotes.com)
- FATE OF PYRUVATE | Biochemart (wordpress.com)
- Glycolysis – Bioscience Notes
