Medical Disclaimer: The information presented on the website is only for academic and study purposes and must not be used for the purpose of diagnosis. If you are not feeling well, please consult with your physician or doctor.
Interesting Science Videos
Specimen Collection
Since COVID-19 is an infectious respiratory tract infection, respiratory material should be collected, at a minimum:
- upper respiratory specimens: nasopharyngeal and oropharyngeal swabs or wash in patients still capable of movement.
- lower respiratory specimens: sputum (if produced) or endotracheal aspirate or bronchoalveolar lavage in patients with more severe respiratory disease.
- Additional clinical specimens can be collected as the COVID-19 virus has been detected in blood and stool.
- In the case of deceased patients, the collection of autopsy material including lung tissue should be considered.
- In recovered patients, paired serum (acute and convalescent) can be favorable to define cases as serological assays become available retrospectively.
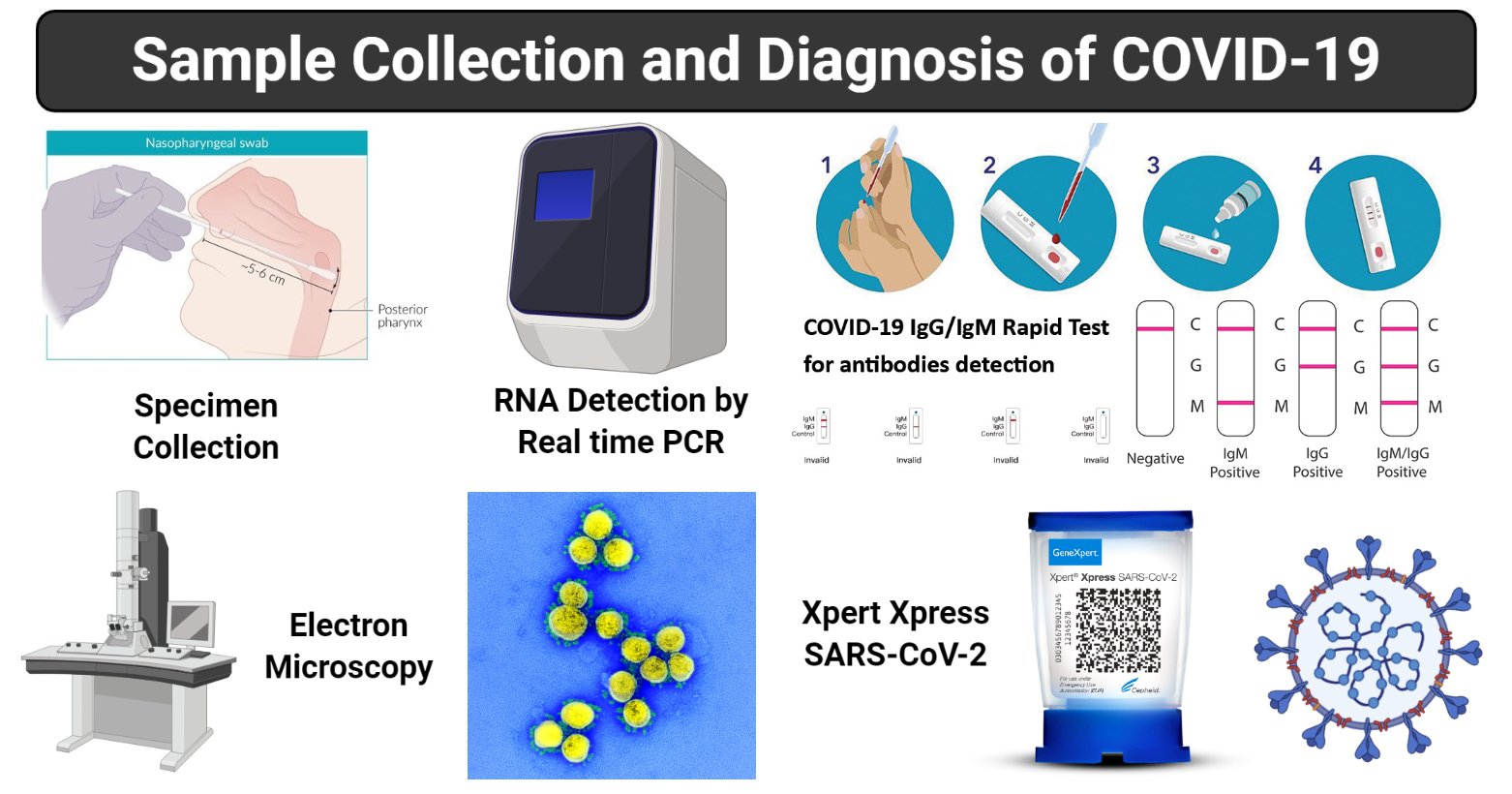
Figure: Sample collection and Diagnosis of COVID-19, created with biorender.com
Packaging and shipping of clinical specimens
- Specimens for virus detection should reach the laboratory as soon as possible after collection.
- Specimens that can be delivered shortly to the laboratory can be stored and shipped at 2-8°C while if a delay is likely to happen, the viral transport medium (VTM) should be used.
- Specimens can be frozen to – 20°C or ideally -70°C and shipped on dry ice if further delays are expected. However, it is essential to avoid repeated freezing and thawing of specimens.
- The transport of potentially COVID-19 virus-containing samples to other countries should follow the UN Model Regulations, and any other essential regulations depending on the mode of transport being used.
Diagnosis of COVID-19
- All the tests related to COVID-19 are to be performed while following the biosafety practices.
Nucleic acid amplification test (NAAT) (RNA Detection)
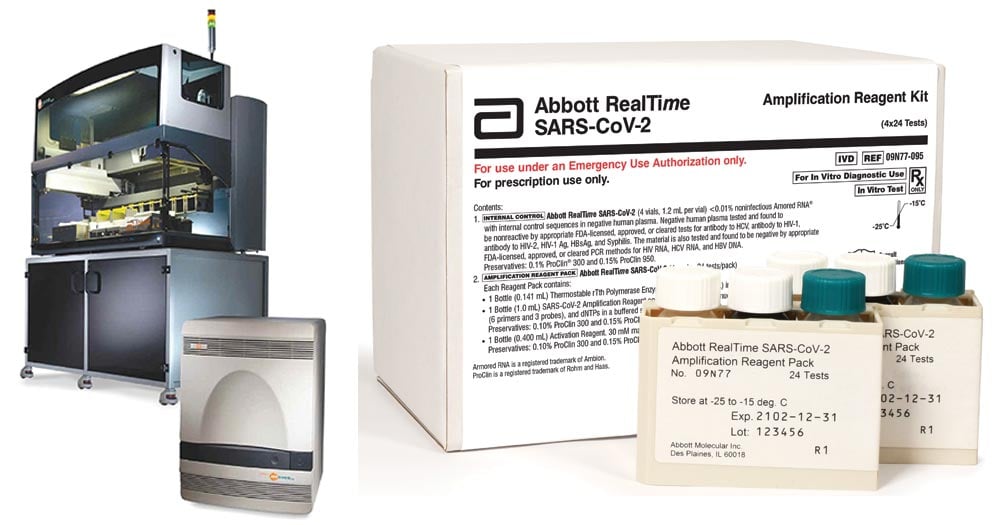
Figure: Abbott RealTime SARS-CoV-2 assay. Image Source: Abbott Laboratories
- Regular confirmation of COVID-19 is based on the detection of particular sequences of viral RNA by NAAT such as real-time reverse-transcription polymerase chain reaction (qRT-PCR).
- The result can be confirmation by nucleic acid sequencing when necessary.
- The viral genes which are targeted include the N, E, S and RdRP genes from samples like nasopharyngeal, oropharyngeal swab and even blood.
- RNA extraction is to be done in a biosafety cabinet in a BSL-2 or equivalent facility. Samples should not be heated before RNA extraction.
- The sample can be further identified by sequencing partial or whole genome of the virus as long as the sequence target is larger or different from the amplicon probed in the NAAT assay used.
Serological Testing
- Serological assays are not routinely used for the diagnosis of human CoV infections due to the lack of commercial reagents.
- When rapid antigen testing and/or molecular assays are neither available nor stable, serology can be used as an additional diagnostic tool.
- Paired serum samples (in the acute and the convalescent phase) should be collected as both IgM and IgG antibodies were detected five days after the onset of infection.
- Serological surveys can aid investigation of an ongoing outbreak and retrospective assessment of the attack rate or extent of an outbreak.
- There are various types of serological test done for diagnosis of coronavirus:
- Complement Fixation test
- ELISA
- Haemagglutination test
- Immunofluorescence, etc.
Viral sequencing
- Viral sequencing can also be used to provide confirmation of the presence of the virus.
- Besides, regular sequencing of a percentage of specimens from clinical cases can be useful to monitor the viral genome mutations that might affect the performance of medical countermeasures, including diagnostic tests.
- Virus whole-genome sequencing can also inform molecular epidemiology studies.
Viral culture (Virus Isolation)
- Virus isolation is not recommended as a routine diagnostic procedure due to the lack of permissive cell lines, time to results, labour and expertise requirements, and the lack of commercial antisera for culture confirmation.
- SARS-CoV and MERS-CoV and SARS-CoV-2 will grow in primary monkey cells and cell lines such as Vero and LLC-MK2. Still, cell culture should not be performed for suspect cases in routine diagnostic laboratories for biosafety reasons.
- However, virus isolation in cell cultures is critical to obtain isolates for characterization and to support the development of vaccines and therapeutic agents.
Rapid antigen tests
- Rapid antigen tests would provide the advantage of fast time to results and low-cost detection of human CoVs, however, they are likely to suffer from reduced sensitivity based on the experience with this method for other respiratory viruses.
- The incorporation of colloidal gold-labeled immunoglobulin G (IgG) as the detection reagent is an approach that may increase the sensitivity of rapid antigen tests for respiratory viruses.
- Monoclonal antibodies, specifically against SARS-CoV-2, have been under preparation. Novel approaches to concentrate antigen, or to amplify the detection phase are needed if these methods are to be used commonly.
COVID-19 IgG/IgM Rapid Test
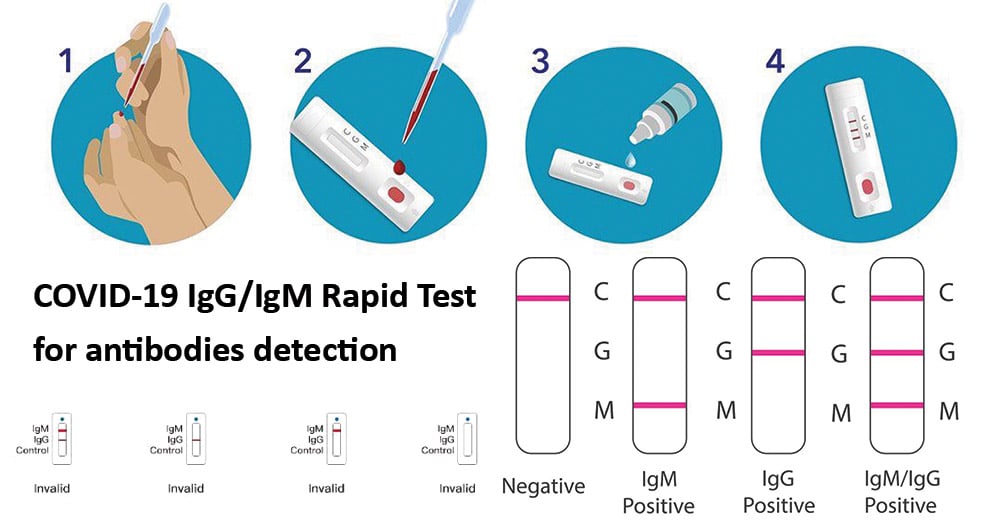
- Qualitative detection of IgM and IgG antibodies against SARS-CoV-2 in serum, plasma (EDTA or citrate), or venipuncture whole blood from individuals suspected of COVID-19.
- Gives results within 2 to 10 minutes.
- Low sensitivity
Electron Microscopy
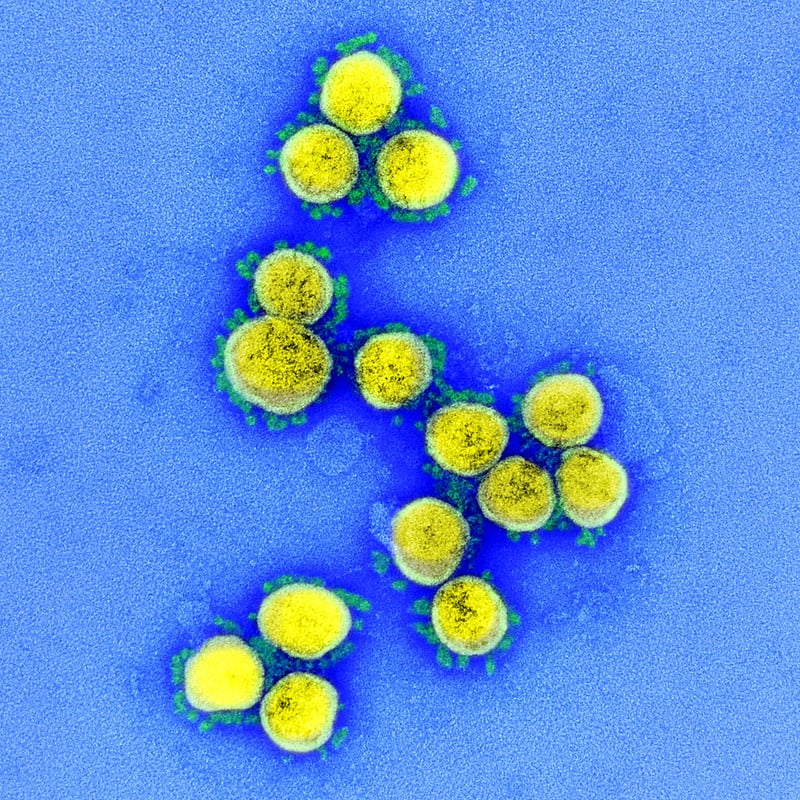
Figure: Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
- Spikes on the surface of the virus can be seen under the electron microscope (which gives the coronavirus its name, meaning ‘crown’).
References
- Laboratory testing for coronavirus disease (COVID-19) in suspected human cases, interim guidance. March 19. World Health Organization. 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117
- Guo Y, Cae Q, Hong Z, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Millitary Medical Reasearch. (2020) 7:11. https://doi.org/10.1186/s40779-020-00240-0
- Adhikari et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious Diseases of Poverty. (2020) 9:29.
Sources
- 11% – https://www.tandfonline.com/doi/full/10.1080/22221751.2020.1745095
- 6% – https://www.researchgate.net/publication/340069714_Laboratory_Diagnosis_of_Emerging_Human_Coronavirus_Infections_-_The_State_of_the_Art
- 4% – https://www.3-14.com/post/covid19-testing
- 2% – https://corporate.cyrilamarchandblogs.com/2020/03/ncovid-19-from-detection-to-a-cure-a-regulatory-overview/
- 2% – https://apps.who.int/iris/rest/bitstreams/1266309/retrieve
- 2% – https://apps.who.int/iris/bitstream/handle/10665/259952/WHO-MERS-LAB-15.1-Rev1-2018-eng.pdf?sequence=1
- 2% – http://www.tnstate.edu/chemistry/Biosafety%20TEST-Final2.pdf
- 1% – https://www.who.int/diseasecontrol_emergencies/publications/idhe_2009_london_sample_collection_healing.pdf
- 1% – https://www.thoracic.org/patients/patient-resources/resources/novel-wuhan-coronavirus.pdf
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4472754/
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1797634/
- 1% – https://www.coursehero.com/file/191777/Homework-4-Outbreak-Investigation-Reading/

Dear Colleagues,
these are wonderful pictures, but all of them are missing a scale bar.
Kind regards,
Mario