Denitrification is the natural conversion of nitrate (NO3–) ions to biologically inert nitrogen (N2) gas via the help of microorganisms.
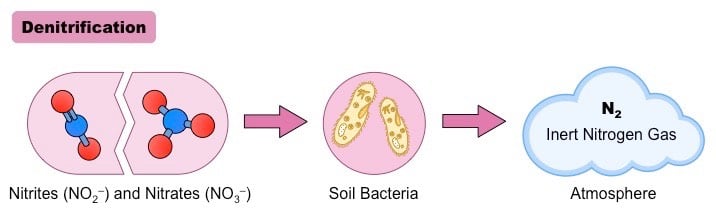
This process helps in retaining bioavailable nitrogen back in the atmosphere. N2, or the dinitrogen gas, is the final end product of interest though other forms of gaseous nitrogen may be released into the environment as a result of denitrification. One such gas is nitrous oxide (N2O), an ozone-depleting greenhouse gas. It is an anaerobic process that occurs mainly in the oxygen-deprived zones of soils, sediments, lakes, and oceans. This process has proved beneficial in wastewater treatment plants and is a good bioremediation tool for extracting nitrogen.
Interesting Science Videos
Nitrogen – Key Element in the Living Beings
Nitrogen among other elements such as carbon, hydrogen, oxygen, and phosphorus, contributes as a major primary nutrient for an organism’s survival due to its presence in many biomolecules like proteins, chlorophyll and most importantly in DNA. Although nitrogen is present abundantly in the atmosphere (approximately 78%) but in an inaccessible form for biological entities. Hence, making this a limiting factor for nitrogen uptake, causing a scarcity among living beings.
Only when dinitrogen gas (N2) is converted to ammonia (NH3) by microbes, which can be easily taken up by the plants (primary producer) to produce sugar and other nutrient resources. To overcome the challenge of conversion from inorganic form ( e.g. nitrate, nitrous oxide, ammonia) to organic forms (e.g. nucleic acids, and proteins), the nitrogen goes through many biological and natural transformation processes like nitrification, nitrogen fixation, denitrification, ammonification, and ammonax. In this article, we will explore one such process of biologically accessible inert nitrogen extraction from the atmosphere.
Denitrification Reaction
The process of denitrification takes place through a set of half-reactions, which are:
NO3– + 2H+ + 2e– → NO2– + H2O
NO2− + 2 H+ + e− → NO + H2O
2NO + 2 H+ + 2 e− → N2O + H2O
N2O + 2 H+ + 2 e− → N2 + H2O
The overall reaction can be represented as:
2NO3− + 10e− + 12H+ → N2 + 6H2O
Denitrifying Microorganisms
- This mainly includes bacteria, archaea, and fungi.
- They are widely spread out in the anoxic zones of soil, sediments, lakes, ocean, and muddy water.
- In anaerobic conditions, these facultative bacteria use oxidized nitrogen compounds such as nitrate (NO3–) as the electron acceptor and produce dinitrogen due to the metabolic oxidoreduction reaction.
- They use various enzymes like reductases to carry out the reduction process.
- Examples include Micrococcus denitrificans, Thiobacillus denitrificans, and some Pseudomonas, Achrobacter, and Serratia species.
Denitrification- Detailed Analysis
To make the nitrogen available for use by higher organisms, atmospheric nitrogen must be made accessible with the help of microorganisms in the surroundings.
- To be readily taken up by the primary producers (plants), soil microbes use the denitrification process to metabolically reduce nitrate compounds to gases like dinitrogen (N2), nitrous oxide (N2O), etc.
- Denitrification usually occurs in the soil when the pores are filled with water causing an anoxic condition.
- This anoxic condition (low supply of oxygen) leads to the bacteria seeking out the oxygen in the nitrates as an electron acceptor in the respiration process.
Denitrification Hotspots
Since the denitrification process is carried out by the microbial species which are present greatly in the topsoil layer, hence higher denitrification activity can be observed here. Beneath the topsoil layer, denitrification is carried out with the aid of plant roots, as they take up oxygen via root cells and water via evapotranspiration. In a study, it’s been observed that denitrification is highest around decomposing living entities. They can also be seen in places with an extended dry period followed by a single rainstorm. Several factors such as the random placement of crop residues, aggregation of organic matter, and distribution of fertilizers influence and stimulate denitrification.
Denitrification Limiting Factors
- Nitrate Concentration – Denitrification process is only carried out in the presence of nitrate compounds, so a lower concentration of nitrate reduces denitrification and supports healthy plant growth. Also, the use of nitrification inhibitors in nitrogen fertilizers limits denitrification.
- Temperature – Optimal denitrifying activity can be observed at 20-35 degree Celsius.
- Soil Moisture – To trigger denitrification, the lower availability of oxygen in the water-logged soil provides a suitable condition, leading to the release of dinitrogen gas into the surroundings.
- Dissolved Carbon Availability – For maximum microbial activity for denitrification, carbon sources such as compost, crop residues, manure, etc, should be readily available to the microorganisms to reduce nitrate to dinitrogen. Lower concentrations of carbon sources lead to the production of nitrous oxide from nitrate as the end product.
Factors Reducing Denitrification
Denitrification can be seen as either detrimental or profitable based on the objectives and the outcome expected. In crop production, the loss of nitrate compounds can lead to lower availability of nitrogen which can hinder plant growth. But in wastewater treatment plants, microbes are specifically employed to reduce nitrate concentration in the water bodies which can cause eutrophication and diseases like cancer, blue baby syndrome, thyroid, fatigue, etc, in humans alike.
- Drainage Systems – To reduce denitrification from the soil, drainage pipes can be placed in the fields to block any water logging, hence maintaining appropriate aerobic conditions.
- Nitrogen Inhibitors – Fertilizers are equipped with nitrification inhibitors, which help in slowing down the conversion of ammonium-N (NH3) to nitrate-N (NO3-), hence increasing the efficiency of fertilizers and reducing denitrification with lower N2O (greenhouse gas) emission.
- Irrigation Practices – To avoid over-wetting of soil, subsurface drip irrigation is employed to reduce denitrification as lower availability of nitrate concentration is present in the soil.
Application of Denitrification in Wastewater Treatment
Denitrification proved to be beneficial for the removal of excess nitrogen compounds from sewage and wastewater. Water bodies are polluted with nitrate compounds due to leaching from crop fields, the use of nitrogen-containing chemicals from residential sewage, and industrial effluents. Wastewater is supplied with additional carbon sources such as methanol, glycerin, acetate, and more, to enhance the denitrifying activity of the bacterial species.
Negative Impact of Denitrification on Human Health
High levels of nitrate in the wastewater may find their way to the aquatic systems which causes harmful effects to humans and aquatic organisms alike. Some of the diseases seen are:
- Birth Defects – Pregnant women consuming water with high levels of nitrate may lead to defects in the organization of neural tubes (formation of brain and spinal cord) in the foetus.
- Methemoglobinemia – Nitrate leads to allosteric changes in the haemoglobin structure to methemoglobin containing ferric ions (Fe3+) causing increased affinity for oxygen and tissue hypoxia due to low delivery of oxygen to the tissues.
- Thyroid Malfunction – Nitrate competitively inhibits the binding of iodine which lowers the level of thyroid hormones causing fatigue, heart-rate elevation, weakness, and dizziness.
- Cancer – Consumption of nitrates may cause endogenous compounds to turn into carcinogens in the colon cells and other body tissues, hence causing cancer.
Eutrophication and Denitrification
Excess nitrate causes eutrophication and algal blooms in marine water bodies allowing microbes with nutrient sources to flourish. They also increase aquatic plant growth which can limit the carbon and nutrient resources to other water organisms. Hence, causing the death of certain fishes, and other organisms.
Conclusion
To maintain the balance of nitrogen availability to all organisms in the environment, the denitrification process is important. Under anaerobic conditions, bacteria enzymatically transform nitrate (NO3–) to dinitrogen (N2) gas via a series of metabolic reactions in the water-logged soil surface. Removal of excess nitrate from the environment helps in restoring nitrogen balance and reducing effects like eutrophication and diseases like cancer in humans. Nitrous oxide (N2O), a byproduct of denitrification, is a greenhouse gas that contributes to the ozone depletion of the stratosphere. But excess denitrification may lead to a reduction in nitrogen availability to the plants, hence hindering plant growth. So a balanced nitrogen cycle is important for restoring nitrogen bioavailability for optimal use.
References
- The Nitrogen Cycle: Processes, Players, and Human Impact – https://www.nature.com/scitable/knowledge/library/the-nitrogen-cycle-processes-players-and-human-15644632/
- Denitrification – http://www.ipni.net/publication/nitrogen-en.nsf/0/668099AE825517CB85257DD600054B8C/$FILE/NitrogenNotes-EN-5.pdf
- Denitrification – https://en.wikipedia.org/wiki/Denitrification
- Anoxic zone – https://www.accessscience.com/content/article/a037400?implicit-login=true
- How Does Nitrogen Enter Our Body? – https://sciencing.com/nitrogen-enter-body-5180380.html
- Denitrifying Bacteria – https://www.vedantu.com/biology/denitrifying-bacteria
- Denitrifying bacteria – https://en.wikipedia.org/wiki/Denitrifying_bacteria
- Liao, Runhua, et al. “Temperature dependence of denitrification microbial communities and functional genes in an expanded granular sludge bed reactor treating nitrate-rich wastewater.” RSC advances 8.73 (2018): 42087-42094.
- Nitrate Water Pollution – https://storymaps.arcgis.com/stories/e7e6ec676acb4cc58d3d35e914efc207
- https://dhs.wisconsin.gov/publications/p02559.pdf
- Methemoglobinemia- https://www.ncbi.nlm.nih.gov/books/NBK537317/
- Ward, M. H., et al. “Nitrate from drinking water and diet and thyroid disorders and thyroid cancer among women.” Epidemiology 18.5 (2007): S169.
