Interesting Science Videos
Structure of Crimean-Congo Hemorrhagic Fever Virus
- Crimean Congo hemorrhagic fever virus falls under the family Bunyaviridae and genus Nairo virus.
- They are spherical particles 90 to 120 nm in diameter with 5-10 nm projections visible on the surface.
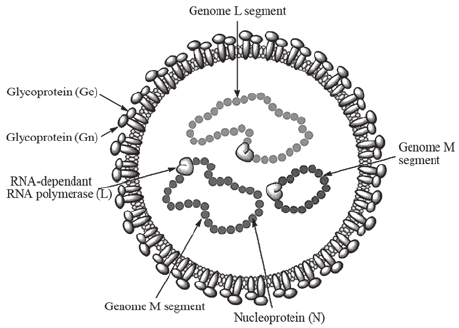
Figure: Crimean‐Congo hemorrhagic fever virus structure. Source: DOI: 10.3892/br.2015.545
- They are enveloped virus which comprises of two glycoproteins (G1 and G2).
- The genome is tripartite, RNA genomes of negative sense, termed the large (L), medium (M) and small (S) segments that are associated with protein to form nucleocapsids.
- The nucleocapsid is surrounded by a lipid-containing envelope.
- The nucleocapsids include the RNA-dependent RNA polymerase (L protein).
Genome of Crimean-Congo Hemorrhagic Fever Virus
- The genome is linear, tripartite, segmented negative-stranded RNA.
- It comprises of three segments: large, medium and small.
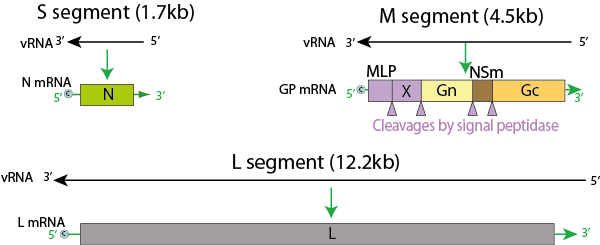
Figure: Genome of Nairovirus, Source: Viral Zone
- L segment is 12164 nucleotides, M segment is 4888 nucleotides and S segment is 1712 nucleotides.
- It encodes for four to six proteins.
- Terminal, complementary, nucleotide sequences are conserved on the L, M, and S segments for viruses.
- The S segment of nairoviruses encodes only a large N protein and has no known nonstructural protein coding information.
- The M segment of the nairovirus seems to encode only G2 and G1.
- Nucleotide sequencing of the L RNA of Crimean-Congo hemorrhagic fever virus revealed the segment is 12164 nucleotides in length and encodes 3944 amino acids.
- The viral RNA dependent RNA polymerase (L) bind to a promoter on each encapsidated segment, and transcribes the mRNA.
- These are capped by L protein during synthesis using cap snatching.
Epidemiology of Crimean-Congo Hemorrhagic Fever Virus
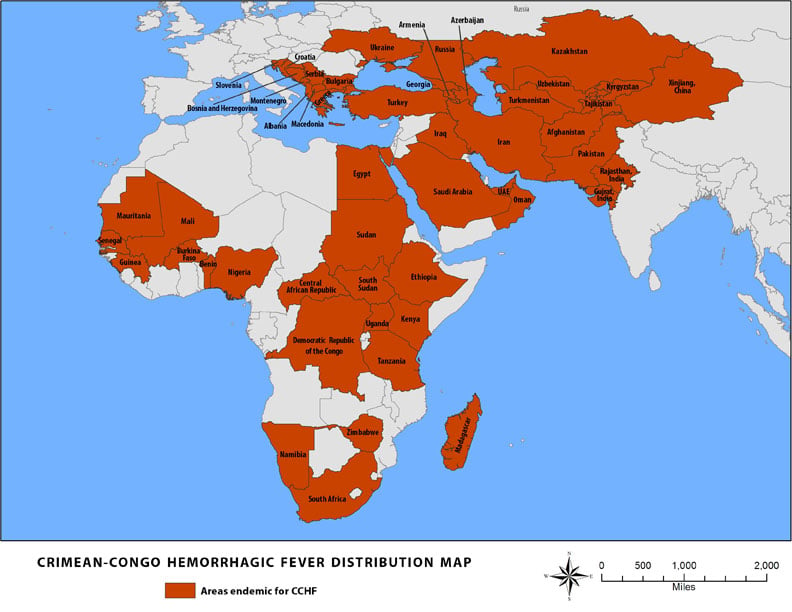
Source: CDC, 2014
- Crimean-Congo hemorrhagic fever (CCHF) virus, of the Nairovirus genus, was first recognized in the Crimean peninsula (in the south of present day Ukraine) in an outbreak of hemorrhagic fever among agricultural workers.
- The same virus was isolated in 1956 from a single patient in present day Democratic Republic of Congo in Kenya, leading to the actual naming.
- Although animal and human can be infected, only the later develop a disease.
- Crimean-Congo hemorrhagic fever is a tick-transmitted viral disease found in Bulgaria, Yugoslavia, the former Soviet Union, China, Iraq, United Arab Emirates, Pakistan, and sub-Saharan Africa. The vector tick is usually of the Hyalomma
Transmission of Crimean-Congo Hemorrhagic Fever Virus
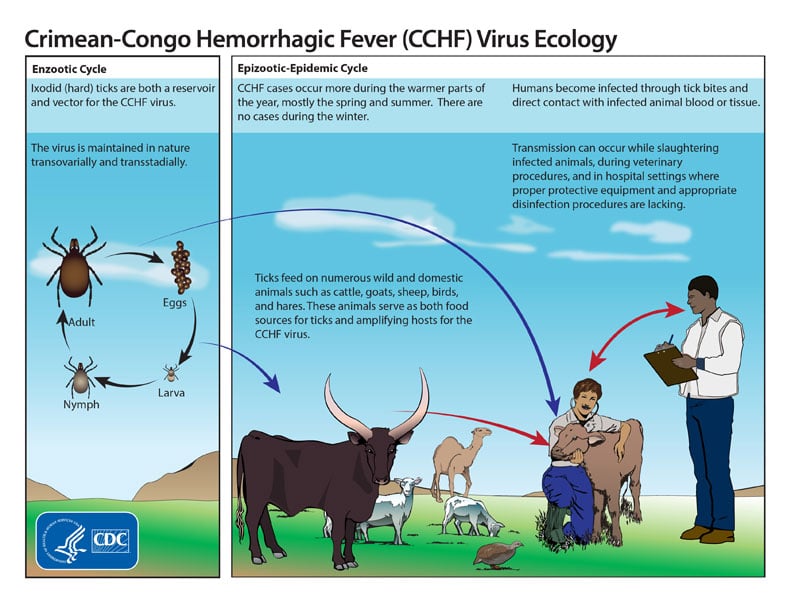
Figure: Life cycle of the Crimean-Congo hemorrhagic fever virus, Source: CDC
- Ixodid (hard) ticks, especially those of the genus, Hyalomma, are both a reservoir and a vector for the CCHF virus.
- Transmission to humans occurs through contact with infected ticks or animal blood.
- CCHF can be transmitted from one infected human to another by contact with infectious blood or body fluids.
- Improper sterilization of medical equipment, reuse of injection needles, and contamination of medical supplies can result in spread of CCHF in hospital premises.
Replication of Crimean-Congo Hemorrhagic Fever Virus
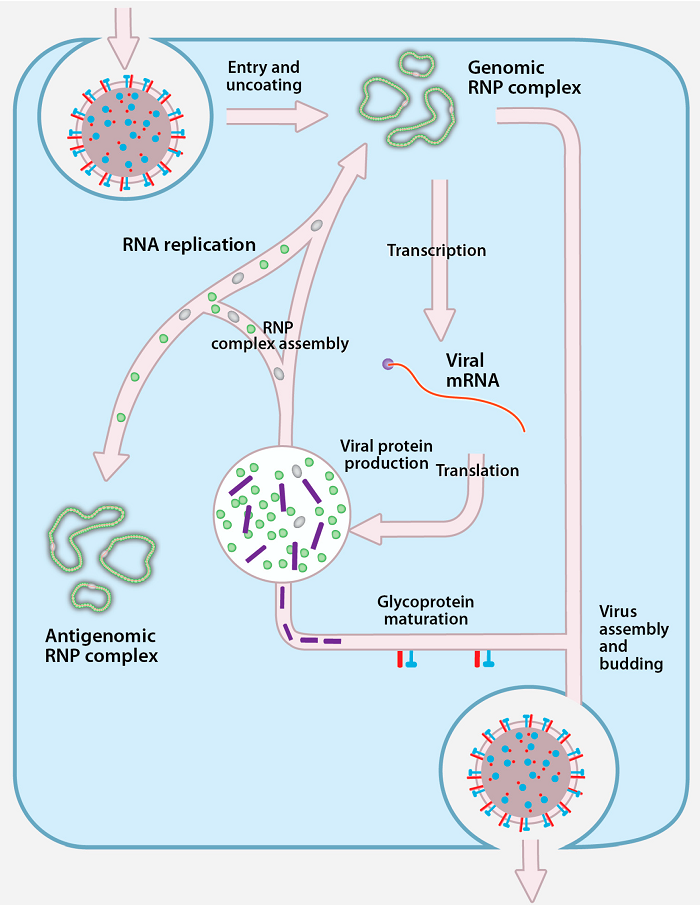
Figure: Replication of Crimean-Congo Hemorrhagic Fever Virus, Source: doi:10.3390/v8040106
- Virus attaches to host receptors though glycoprotein, and is endocytosed into vesicles in the host cell.
- Fusion of virus membrane with the vesicle membrane results in the release of ribonucleocapsid in the cytoplasm.
- The RNA dependent RNA polymerase (RdRp) complex initiates transcription by binding to the leader sequence in 3′ of the genomic negative strand RNA and viral mRNAs are capped in the cytoplasm.
- During replication the RNA dependent RNA polymerase complex binds to the leader sequence on the encapsidated (-)RNA genome, and starts replication.
- The antigenome is concomitantly encapsidated during replication and replicate to give rise to negative sense genome.
- Nucleocapsids assembled induce formation of a membrane curvature in the host cell membrane and wrap up in the forming bud at golgi apparatus releasing the enveloped virion by exocytosis.
Pathogenesis of Crimean-Congo Hemorrhagic Fever
Source: https://doi.org/10.1089/vbz.2012.1061
- The gut of the vector is infected initially, and after a few days or weeks the virus appears in the saliva.
- When the vector takes a blood meal, the infective saliva enters the small capillaries or lymphatics of the human or other vertebrate host.
- An incubation period of a few days ensues, after which the vertebrate host develops viremia.
- The host becomes febrile, manifesting the more serious signs and symptoms that are characteristic of the infecting virus.
- A typical humoral immune response leads to cessation of viremia and clinical recovery in most cases, with immunoglobulin M (IgM) predominating initially, followed by immunoglobulin G (IgG) and the host recovers unless a specific target organ is affected.
- The target organ is the liver and vascular endothelium in Crimean-Congo hemorrhagic fever.
- This further leads to hemostatic failure by stimulating platelet aggregation and degranulation, with subsequent activation of the intrinsic coagulation cascade.
- The proinflammatory cytokines are key regulators in the pathogenesis and mortality of patients with CCHF.
- Levels of Interleukin (IL)-6 and Tumor Necrosis Factor (TNF)-α are shown to be significantly higher in patients with fatal CCHF
Clinical manifestations of Crimean-Congo Hemorrhagic Fever
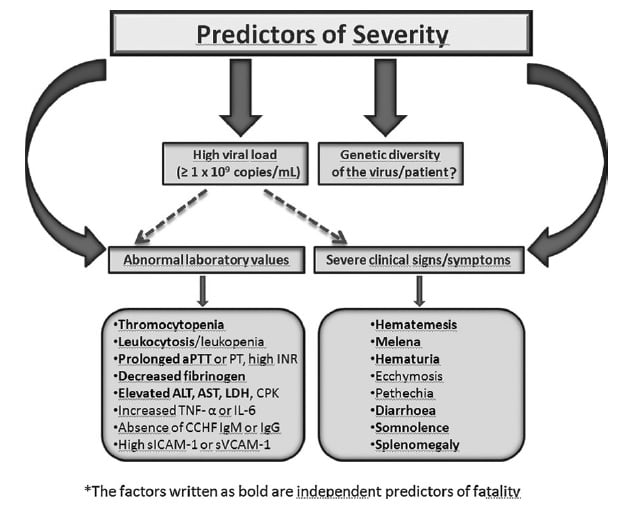
Source: https://doi.org/10.1089/vbz.2012.1061
- Following an incubation period of 3 to 21 days, a non-specific febrile illness of abrupt onset develops.
- The initial signs and symptoms include headache, high fever, back pain, joint pain, stomach pain, nausea and non bloody diarrhea.
- Red eyes, a flushed face, a red throat, and petechiae (red spots) on the palate are common.
- This is accompanied by hypotension, relative bradycardia, tachypnea, conjunctivitis, pharyngitis, and cutaneous flushing or rash.
- Symptoms may also include jaundice, and in severe cases, changes in mood and sensory perception.
- The hemorrhagic phase is generally short and has a rapid course with signs of progressive hemorrhage and diathesis which include petechiae, conjunctival hemorrhage, epistaxis, hematemesis, hemoptysis, and melena.
- Internal bleeding, including retroperitoneal and intracranial haemorrhage, may occur..
- Hepatosplenomegaly may be present and in severe cases, death occurs as a result of multiorgan failure, disseminated intravascular coagulation, and circulatory shock.
Diagnosis of Crimean-Congo Hemorrhagic Fever Virus
- Virus Isolation- intracranial inoculation of suckling mice is thought to be the most sensitive system available for virus isolation. However, several sensitive cell culture systems are available such as vero, LLC-MK2 and BHK-21 cell lines.
- Immunohistochemical staining can also show evidence of viral antigen in formalin-fixed tissues.
- Detection of antibody (IgG and IgM) by ELISA.
- Detection of the viral antigen (ELISA antigen capture), viral RNA sequence (RT-PCR) in the blood or in tissues collected from a fatal case and virus isolation.
Treatment of Crimean-Congo Hemorrhagic Fever
- Supportive treatment is given which include fluid balance and correction of electrolyte abnormalities, oxygenation and hemodynamic support.
- The virus is sensitive in vitro to the antiviral drug ribavirin and often administered in both IV and oral forms to patients with the disease with apparent benefit.
Prevention and control of Crimean-Congo Hemorrhagic Fever Virus
- There is no safe and effective vaccine currently available for human use.
- In case of known direct contact with blood or secretions of a probable or confirmed case such as needlestick injury or contact with mucous membranes such as eye or mouth, the recommended procedure is to carry out baseline blood studies and start the patient on oral ribavirin as post exposure prophylaxis.
- Use of insect repellent on exposed skin and clothing.
- Wearing gloves and other protective clothing is recommended.
- Avoiding contact with the blood and body fluids of livestock or humans who show symptoms of infection.
- Use of proper infection control precautions to prevent occupational exposure.
