Interesting Science Videos
Habitat of Corynebacterium diphtheriae
- Corynebacterium diphtheriae is found in nasopharynx as well as in skin lesions, which actually represent a reservoir for the spread of diphtheria.
- Also found in mouth, throat, nose, skin and wound of infected person.
- Animals do not easily contract diphtheria.
- Patients carry toxigenic organism upto 3 months after infection.
Morphology of Corynebacterium diphtheriae
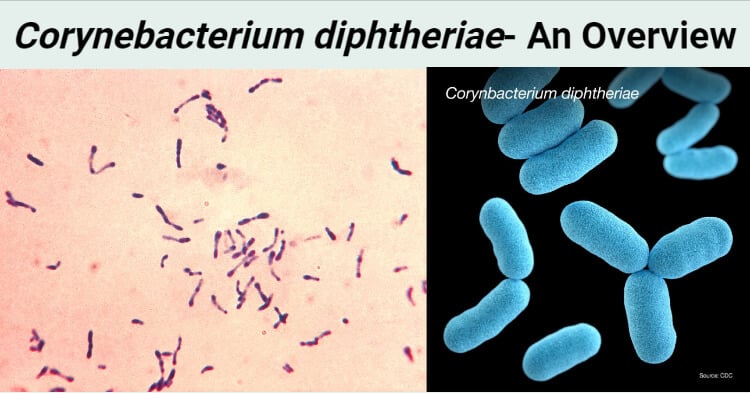
- Corynebacterium diphtheriae are gram +ve rod shaped bacteria.
- Club shaped
- Irregular swelling at one end or both end gives them the “Club Shaped” appearance.
- Thin and slender, long, curved
- Also known as Kleb’s-Loeffler Bacillus.
- 3 µm to 5 µm x 0.5 µm to 0.8 µm in diameter
- Non-capsulated
- Non-sporing
- Non-motile
- Chinese letter arrangement, V or L shaped
- Pleomorphic (Changes in shape and size of the bacteria)
- Seen in pairs or in small groups, appears in clusters
- Arranged in various angles to each other.
- Forms granules (metachromatic granules)
- Granules are the store house of energy
- Lipid rich cell wall contains mesodiaminopimelic acid, arabino-galactam polymers and short chain mycolic acid.
- Pili present
- Alberts’s stain and Ponder’s stain shows metachromatic granules formed in the polar regions
- Appears green in Alberts’s stain along with bluish black metachromatic granules at the poles.
Genome of Corynebacterium diphtheriae
- Contains 2389 Genes
- Protein encoding genes: 2272
- Have 69 Structural RNA gene
- Genome Length: 2,488,635 nucleotide
- 87% are coding
- G+C Content: 53%
- No plasmid
- Contains Pathogenicity Islands (PAI’s)
Cultural Characterisitics of Corynebacterium diphtheriae
- Corynebacterium diphtheriae grows well on simple nutrient agar and agar containing blood at the mesophilic temperature range. The growth, however, is not observed on enteric agar formulation and on MacConkey agar.
- The growth of C. diphtheriae on agar media is accomplished by growing it on media containing compounds like nalidixic acid and colistin sulfate that are inhibitory to gram-negative bacteria.
- Like most corynebacteria, C. diphtheriae also grows well at 37 °C or within the mesophilic temperature range.
- Some strains of C. diphtheriae are facultative anaerobes that grow well in a CO2-enriched atmosphere.
- The most important culture media used for the isolation of C. diphtheriae is the Sheep Blood Agar (SBA). Other selective media include cystine-tellurite blood agar (CTBA) or freshly prepared Tinsdale medium.
- The tellurite present in the medium inhibits the growth of other noncorynebacterium species, but some strains of C. diphtheriae are sensitive to potassium tellurite and require the SBA agar.
- For the direct culturing of C. diphtheriae, Tinsdale medium is the best medium, but the medium has a short shelf life.
- The identification of C. diphtheriae on Tinsdale medium is efficient in the identification of C. diphtheriae as the bacteria shows both tellurite reductase and cystinase activity.
- C. diphtheriae belongs to the Hazard group 2 organisms which might require Containment Level 3 conditions for processing in some cases due to aerosol production.
- Growth on serum-based media like Loeffler slants produce colonies with pleomorphic rods, some of which might be club-shaped. The cells might have a beaded appearance due to the terminal reddish-purple polyphosphate granules.
- Members of C. diphtheriae have been divided into four different biotypes based on their colony morphology on different agar medium.
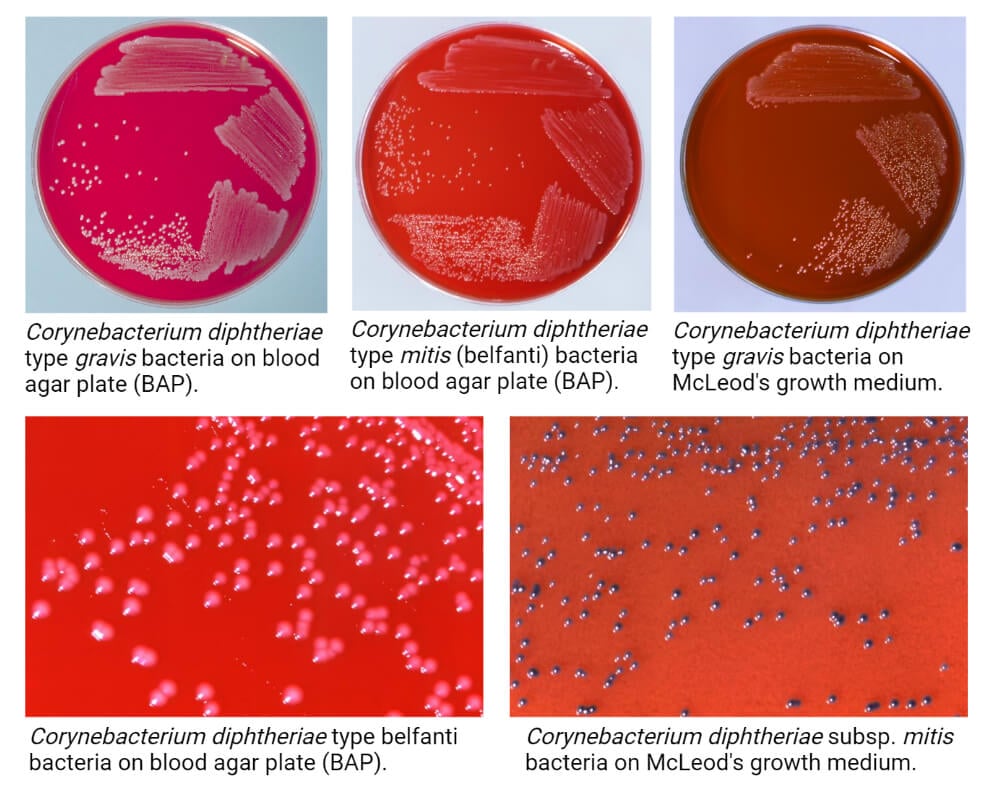
- The following are some cultural characteristics of C. diphtheriae on different culture media:
1. Tellurite Medium
- The colony morphology of different biotypes of C. diphtheriae on the tellurite medium might be different.
- C. diphtheriae biotype biovar gravis forms dull, grey-coloured opaque colonies that have an average diameter of 2 mm. The surface of the colonies is matt, friable that tends to break into small segments when touched with a wire.
- C. diphtheriae biotype biovar mitis produces opaque grey or black colonies ranging in size between 1.5-2 mm in diameter. The colonies have entire edge and glossy smooth surface. The variation in size is common in this biotype.
- C. diphtheriae biotype biovar intermedius forms small grey-coloured shiny colonies that are comparatively smaller in size (0.5-1.0 mm in diameter). The colonies are discrete and translucent on the surface.
- C. diphtheriae biotype biovar belfanti produces grey or black-coloured colonies of 1.5-2.0 mm diameter. The colonies have an entire edge and glossy smooth surface.
- The black coloured colonies of C. diphtheriae on tellurite agar are due to the tellurite reductase activity of the bacteria. Besides, these can also produce a brown halo around the colonies due to the cystinase activity.
2. Sheep Blood Agar
- Small grey to black-coloured colonies is observed on the sheep blood agar. The colonies usually range between 1-2 mm in diameter.
- C. diphtheriae exhibits hemolytic activity on blood agar, which might differ in different biotypes.
- C. diphtheriae biotype biovar gravis is non-hemolytic and thus doesn’t cause clearing of the media.
- The rest three biovars of the species; mitis, intermedius and belfanti produce colonies that exhibit a small zone of β-hemolysis on the agar.
Click here for Biochemical Test of Corynebacterium diphtheriae
Pathogenesis of Corynebacterium diphtheriae
- In nature, C diphtheriae occurs in the respiratory tract, in wounds, or on the skin of infected persons or normal carriers.
- It is spread by droplets or by contact to susceptible individuals; the bacilli then grow on mucous membranes or in skin abrasions, and those that are toxigenic start producing toxin.
- Diphtheria toxin is the major virulence factor of C. diphtheria and is a heat-labile, single-chain, three domain polypeptide (62 kDa) that can be lethal in a dose of 0.1 μg/kg body weight.
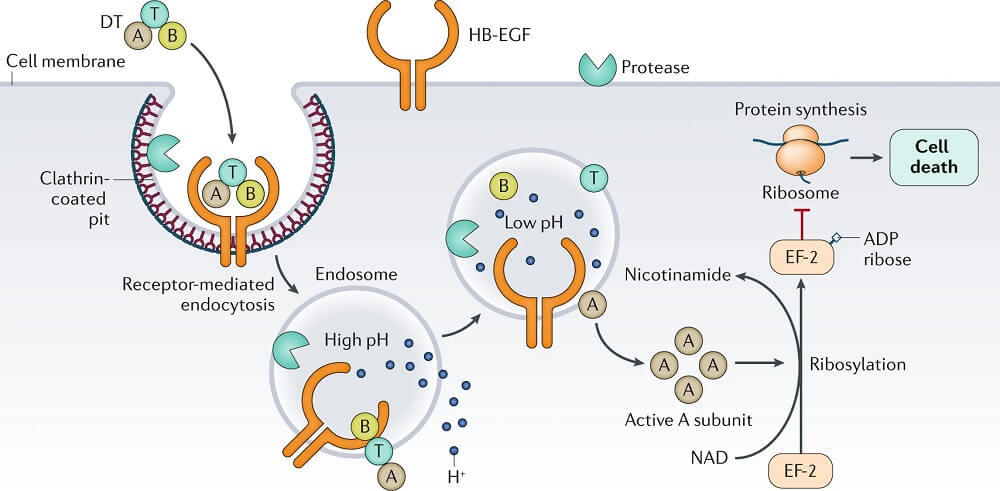
- The tox gene that codes for the exotoxin is introduced into strains of C. diphtheriae by a lysogenic bacteriophage, β-phage.
- Two processing steps are necessary for the active gene product to be secreted: (1) proteolytic cleavage of the leader sequence from the Tox protein during secretion from the bacterial cell and (2) cleavage of the toxin molecule into two polypeptides (A and B) that remain attached by a disulfide bond.
- Three functional regions exist on the toxin molecule: a catalytic region on the A subunit and a receptor-binding region and a translocation region on the B subunit.
- The receptor for the toxin is heparin-binding epidermal growth factor, which is present on the surface of many eukaryotic cells, particularly heart and nerve cell.
- The binding of the receptor domain to host cell membrane proteins CD-9 and heparin-binding epidermal growth factor (HB-EGF) triggers the entry of the toxin into the cell through receptor-mediated endocytosis.
- Acidification of the translocation domain within a developing endosome leads to creation of a protein channel that facilitates movement of Fragment A into the host cell cytoplasm.
- The A subunit then terminates host cell protein synthesis by inactivating elongation factor-2 (EF-2) which is required for translocation of polypeptidyl-transfer RNA from the acceptor to the donor site on the eukaryotic ribosome.
- Toxin Fragment A inactivates EF-2 by catalyzing a reaction that yields free nicotinamide plus an inactive adenosine diphosphate-ribose-EF-2 complex (ADP-ribosylation).
- Because the turnover of EF-2 is very slow and approximately only one molecule per ribosome is present in a cell, it has been estimated that one exotoxin molecule can inactivate the entire EF-2 content in a cell, completely terminating host cell protein synthesis.
- It is assumed that the abrupt arrest of protein synthesis is responsible for the necrotizing and neurotoxic effects of diphtheria toxin.
- Toxin synthesis by lysogenized C diptheriae is regulated by a chromosomally encoded element, diphtheria toxin repressor (DTxR) which is activated in the presence of high iron concentrations, and can bind to the toxin gene operator and prevent toxin production.
- Low iron concentration and other factors such as osmolarity, amino acid concentrations and pH, however enhance the production of toxin.
Clinical manifestations of Corynebacterium diphtheriae
Respiratory diphtheria
- Diphtheria toxin is absorbed into the mucous membranes and causes destruction of epithelium and a superficial inflammatory response.
- The symptoms of diphtheria involving the respiratory tract develop after 2 to 4 days incubation period.
- Organisms multiply locally on epithelial cells in the pharynx or adjacent surfaces and initially cause localized damage as a result of exotoxin activity.
- The onset is sudden, with malaise, sore throat, exudative pharyngitis, and a low-grade fever.
- The exudate evolves into a thick pseudomembrane composed of bacteria, lymphocytes, plasma cells, fibrin, and dead cells that can cover the tonsils, uvula, and palate and can extend up into the nasopharynx or down into the larynx
- The regional lymph nodes in the neck enlarge, and there may be marked edema of the entire neck, with distortion of the airway, often referred to as “bull neck” clinically.
- In critically ill patients, cardiac (necrosis of heart muscles) and neurologic complications (demylenation) is most significant.
- Neurotoxicity is proportional to the severity of the primary disease, which is influenced by the patient’s immunity.
- The majority of patients with severe primary disease develop neuropathy, initially localized to the soft palate and pharynx, later involving oculomotor and ciliary paralysis, with progression to peripheral neuritis.
Cutaneous diphtheria
- Cutaneous diphtheria is acquired through skin contact with other infected persons.
- The organism colonizes the skin and gains entry into the subcutaneous tissue through breaks in the skin.
- A papule develops first and then evolves into a chronic, non-healing ulcer, sometimes covered with a grayish membrane
- Systemic signs can develop.
Laboratory diagnosis of Corynebacterium diphtheriae
Specimen
- Dacron swabs from the nose, throat, or other suspected lesions must be obtained before antimicrobial drugs are administered.
- Swabs should be collected from beneath any visible membrane.
- The swab should then be placed in semisolid transport media such as Amies.
Microscopy
- Smears stained with alkaline methylene blue or Gram stain show beaded rods in typical arrangement.
- Cells often contain metachromatic granules (polymetaphosphate), which stain bluish-purple with methylene blue.
- In Gram staining, purple coloured beaded rods, angular and palisade arrangements that creates a ‘Chinese character’ effect is observed.
Culture
- Specimens should be inoculated to a blood agar plate and a selective medium such as a tellurite plate (eg: cystine-tellurite blood agar [CTBA] or modified Tinsdale’s medium) and incubated at 37°C in 5% CO2.
- Non β hemolytic region on blood agar.
- Tellurite inhibits the growth of most upper respiratory tract bacteria and gram-negative rods and is reduced by C. diphtheriae, producing characteristic gray to black color on agar.
- Degradation of cysteine by C. diphtheriae cysteinase activity produces a brown halo around the colonies.
- Tinsdale medium is the best medium for recovering C. diphtheriae in clinical specimens, but it has a short shelf life and requires addition of horse serum.
Biochemical testing
Catalase – positive
Oxidase – negative
Cystinase production – positive
Pyrazinamidase activity – positive
Carbohydrate fermentation
Glucose – positive
Maltose – positive
Sucrose – negative
Trehalose – negative
Urease – negative
Nitrate reduction – positive
Gelatin liquefaction – negative
Toxigenicity Testing
- All isolates of C. diphtheriae should be tested for the production of exotoxin.
- The gold standard for detection of diphtheria toxin is an in-vitro immunodiffusion assay by Elek-Ouchterlony immunodiffusion test (Elek test).
- An alternative method is detection of the exotoxin gene using a polymerase chain reaction (PCR) based nucleic acid amplification method.
- This test can detect the tox gene in clinical isolates and directly in clinical specimens (e.g., swabs from the diphtheritic membrane or biopsy material).
- A positive culture result confirms a positive PCR assay.
- A negative culture result after antibiotic therapy along with a positive PCR assay result suggests that the patient probably has diphtheria.
- Although this test is rapid and specific, strains in which the tox gene is not expressed can give a positive signal.
- Enzyme-linked immunosorbent assays can be used to detect diphtheria toxin from clinical C diphtheria isolates.
- An immunochromatographic strip assay allows detection of diphtheria toxin in a matter of hours.
- However ELISA and immunochromatographic assay are not widely used.
Antibody detection
- Measurement of antibodies to diphtheria toxin in serum collected before administration of antitoxin may support the diagnosis when cultures are negative.
Treatment of Corynebacterium diphtheriae
- The early administration of specific antitoxin against the toxin formed by the organisms at their site of entry and multiplication is done promptly.
- The antitoxin should be given intravenously on the day the clinical diagnosis of diphtheria is made and need not be repeated.
- Intramuscular injection may be used in mild cases.
- Diphtheria antitoxin will only neutralize circulating toxin that is not bound to tissue.
- Antimicrobial drugs (penicillin, macrolides) inhibit the growth of diphtheria bacilli.
- Although these drugs have virtually no effect on the disease process, they arrest toxin production and assist public health efforts.
- Erythromycin may be preferred to penicillin for elimination of the bacilli from the throat, particularly in treatment of persistent carriers.
- Some strains are tolerant to the bactericidal action of penicillins, and treatment of complicated infections should contain an association with an aminoglycoside.
- Patients should be placed in strict isolation, nursed by staff whose immunization history is documented and have daily platelet counts and electrocardiography.
Prevention and control of Corynebacterium diphtheriae
- Active immunization in childhood with diphtheria toxoid yields antitoxin levels that are generally adequate until adulthood.
- Diphtheria toxoids are commonly combined with tetanus toxoid (Td) and with a cellular pertussis vaccine (DaPT) as a single injection to be used in initial immunization of children (three doses in the first year of life, 15–18 months of age and 4–6 years of age).
- Young adults should be given boosters of toxoid because toxigenic diphtheria bacilli are not sufficiently prevalent in the population to provide the stimulus of subclinical infection with stimulation of resistance.
- Levels of antitoxin decline with time, and many older persons have insufficient amounts of circulating antitoxin to protect them against diphtheria.
- The principal aims of prevention are to limit the distribution of toxigenic diphtheria bacilli in the population and to maintain as high a level of active immunization as possible.
- Bed rest, isolation to prevent secondary spread, and maintenance of an open airway in patients with respiratory diphtheria are all important.
References
Chaudhary A, Pandey S. Corynebacterium Diphtheriae. [Updated 2021 Jun 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559015/.
Sharma, N.C., Efstratiou, A., Mokrousov, I. et al. Diphtheria. Nat Rev Dis Primers 5, 81 (2019). https://doi.org/10.1038/s41572-019-0131-y.
Murphy JR. Corynebacterium Diphtheriae. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 32. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7971/
Lamichhane A, Radhakrishnan S. Diphtheria. [Updated 2021 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560911/
Hoskisson PA. Microbe Profile: Corynebacterium diphtheriae – an old foe always ready to seize opportunity. Microbiology (Reading). 2018;164(6):865-867. doi:10.1099/mic.0.000627
Edwards D, Kent D, Lester C, et al. Transmission of toxigenic Corynebacterium diphtheriae by a fully immunised resident returning from a visit to West Africa, United Kingdom, 2017 [published correction appears in Euro Surveill. 2018 Oct;23(42):]. Euro Surveill. 2018;23(39):1700681. doi:10.2807/1560-7917.ES.2018.23.39.1700681
Trost E, Blom J, Soares Sde C, et al. Pangenomic study of Corynebacterium diphtheriae that provides insights into the genomic diversity of pathogenic isolates from cases of classical diphtheria, endocarditis, and pneumonia. J Bacteriol. 2012;194(12):3199-3215. doi:10.1128/JB.00183-12
Hoskisson PA. Microbe Profile: Corynebacterium diphtheriae – an old foe always ready to seize opportunity. Microbiology (Reading). 2018;164(6):865-867. doi:10.1099/mic.0.000627
Barksdale L. Corynebacterium diphtheriae and its relatives. Bacteriol Rev. 1970;34(4):378-422. doi:10.1128/br.34.4.378-422.1970
Rosana Y, Prilandari LI, Ajisman R, Hartono TS, Yasmon A. Detection of toxin-producing Corynebacterium diphtheriae from throat swabs of diphtheria patients using duplex real-time PCR. Iran J Microbiol. 2020;12(6):508-515. doi:10.18502/ijm.v12i6.5024
