Competitive ELISA is a technique used to estimate antibodies present in a specimen, such as serum.
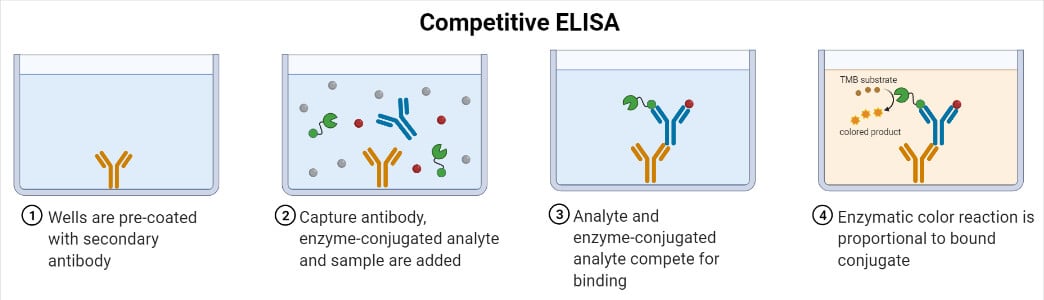
- The principle of the test is that two specific antibodies, one conjugated with enzyme and the other present in test serum (if serum is positive for antibodies), are used.
- Competition occurs between the two antibodies for the same antigen.
- The appearance of color indicates a negative test (absence of antibodies), while the absence of color indicates a positive test (presence of antibodies).
- The central event of competitive ELISA is a competitive binding process executed by the original antigen (sample antigen) and add-in antigen.
- The procedures of competitive ELISA are different in some respects compared with other forms of ELISA.
Interesting Science Videos
Steps/Process of Competitive ELISA
- Primary antibody (unlabeled) is incubated with sample antigen.
- Antibody-antigen complexes are then added to well plates which are pre-coated with the same antigen.
- Unbound antibody is removed by washing the plate. (The more antigen in the sample, the less antibody will be able to bind to the antigen in the well, hence “competition.”)
- The secondary antibody that is specific to the primary antibody and conjugated with an enzyme is added.
- A substrate is added, and the remaining enzymes elicit a chromogenic or fluorescent signal.
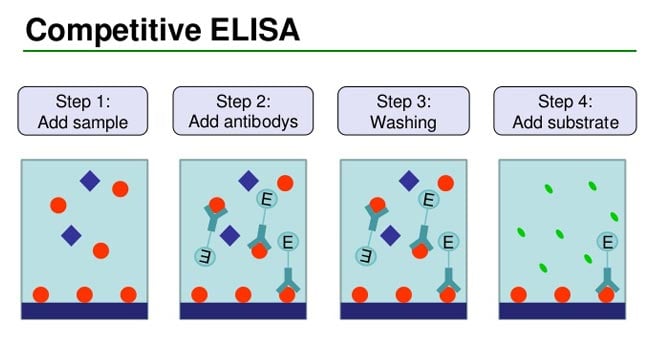
Competitive ELISA Results
- In this test, microtiter wells are coated with antigen.
- The sera to be tested are added to these wells and incubated at 37°C and then washed.
- If antibodies are present in the test serum, antigen–antibody reaction occurs.
- The antigen– antibody reaction is detected by adding enzyme-labeled-specific antibodies.
- In a positive test, no antigen is left for these antibodies to act.
- Hence, the antibodies remain free and are washed away during the process of washing.
- When substrate is added, no enzyme is available to act on it.
- Therefore, positive result is indicated by absence of color reaction.
- In a negative test, in which no antibodies are present in the serum, antigen in the coated wells is available to combine with enzyme-conjugated antibodies and the enzyme acts on the substrate to produce color.
Advantages of Competitive ELISA
- High specificity, since two antibodies are used and the antigen/analyte is specifically captured and detected
- Suitable for complex samples, since the antigen does not require purification prior to measurement
- Flexibility and sensitivity, since both direct and indirect detection methods can be used
Protocol of Competitive ELISA
For most applications, a polyvinylchloride (PVC) microtiter plate is best.
- Add 50 μL of diluted primary antibody (capture) to each microtiter well. Allow to incubate for 4 hrs. at room temperature or 4°C overnight.
Note: If a purified capture antibody is not available, plate should first be coated with a purified secondary antibody, directed against the host of the capture antibody according to the following procedure:
- Bind the unlabeled secondary antibody to the bottom of each well by adding approximately 50 μL of antibody solution to each well (20 μg/mL in Phosphate buffered saline (PBS).
- Incubate the plate overnight at 4°C to allow complete binding.
- Add primary capture antibody (as above).
- Wash the wells twice with PBS. The antibody solution washes can be removed by flicking the plate over a suitable container.
- The remaining sites for protein binding on the microtiter plate must be saturated by incubating with blocking buffer. Fill the wells to the top with 3% BSA/PBS with 0.02% sodium azide. Incubate for 2 hrs. to overnight in a humid atmosphere at room temperature.
- Wash wells twice with PBS.
- Add 50 μL of the standards or sample solution to the wells. All dilutions should be done in the blocking buffer (3% BSA/PBS with 0.05% Tween-20).
Note: Sodium azide is an inhibitor of horseradish peroxidase. Do not include sodium azide in buffers or wash solutions, if an HRP-labeled conjugate will be used for detection.
- Add 50 μL of the antigen-conjugate solution to the wells (the antigen solution should be titrated). All dilutions should be done in the blocking buffer (3% BSA/PBS with 0.05% Tween-20). Incubate for at least 2 hrs. at room temperature in a humid atmosphere.
- Wash the plate four times with PBS.
- Add substrate. After suggested incubation time has elapsed, optical densities at target wavelengths can be measured on an ELISA reader.
Competitive ELISA Animation

