Cefsulodin-Irgasan-Novobiocin Agar (CIN Agar), also known as Yersinia Selective Agar, is a selective and differential culture medium used to selectively isolate and identify Yersinia spp., specifically strains of Yersinia enterocolitica.
This medium was first formulated and described by Schiemann D.A. in 1979. The Schiemann’s CIN medium contained cefsulodin, irgasan, novobiocin, crystal violet, and bile salts as the major selective components besides other nutrient sources. Later, a few components are modified and amounts are adjusted to increase the efficiency of the medium. (The original composition of the CIN medium is described later below.)
Interesting Science Videos
Objective of CIN Agar
To selectively isolate and identify Yersinia enterocolitica strains from fecal samples (clinical samples) and food samples.
Principle of CIN Agar
The CIN medium contains different inhibitory components and antibiotics that suppress the growth of Gram-positive and many Gram-negative bacteria; additionally, the presence of an indicator allows for the presumptive identification of Yersinia enterocolitica colonies.
Crystal violet and sodium deoxycholate (bile salt) present in the medium suppress the growth of Gram-positive bacteria. Irgasan further enhances the suppression of Gram-positive bacterial and fungal growth. The combination of antibiotics like cefsulodin, irgasan, and novobiocin suppresses the growth of many Gram-negative and Gram-positive bacterial growths except Yersinia enterocolitica and few other bacteria, selectively allowing Yersinia enterocolitica to overgrow other competitors.
The mannitol present in the medium is readily fermentable by Yersinia spp. producing organic acids as a byproduct. These produced acids will reduce the pH of the medium which is detected by the neutral red incorporated in the medium as a pH indicator. Yersinia enterocolitica strains are capable of fermenting mannitol and producing acids; hence, they produce characteristic ‘bull’s-eye’ colonies: colonies containing a central dark red/pink zone surrounded by an outer translucent zone.
Composition of CIN Agar
Original Composition Described by Schiemann D.A
The composition of Schiemann’s CIN agar as described by Schiemann D.A in “SCHIEMANN. D. A. 1979. Synthesis of a selective agar medium for Yersinia enterocolitica. Can. J. Microbiol. 25: 1298- 1304” is tabulated below:
Components and Quantity
Special Peptone (Oxoid) – 20 grams
Yeast Extract (Difco) – 2 grams
Mannitol – 20 grams
Pyruvic Acid (Sodium salt) – 2 grams
Sodium Chloride – 1 gram
Magnesium sulfate.7H2O – 10 milligram
(10 mL of 0.1% stock solution)
Agar (Oxoid No. 4) – 12 grams
Irgasan DP300 – 10 mL of 0.04% in 95% ethanol
Bile salts (Difco) – 2 grams in 200 mL distilled water
NaOH – 1 mL of 5N NaOH solution
Neutral Red – 10 mL of 3 mg/mL solution
Crystal Violet – 10 mL of 0.1 mg/mL solution
Cefsulodin – 10 mL of 1.5 mg/mL solution
Novobiocin – 10 mL of 1.5 mg/mL solution
Final pH (Adjusted by 5N NaOH) – 7.4
(Reference: Schiemann D. A., 1979, Synthesis of a selective agar medium for Yersinia enterocolitica. Can. J. Microbiol., 25: 1298. doi: 10.1139/m79-205. PMID: 540256.)
Modern Composition in use as per HiMedia
Most of the company supply CIN Agar Medium as CIN Agar base and supplement material. The agar base contains all the components except antimicrobial components viz. cefsulodin, irgasan, and novobiocin, which are supplied separately as selective supplements as they can’t be autoclaved like other components. HiMedia laboratories also supply in this way. They provide a CIN Agar base called Yersinia Agar Base and CTN Selective Supplement. Mixing these two products in the correct proportion will form CIN Agar for selective isolation of Y. enterocolitica.
Components of CIN Agar per 1000 mL
Peptone – 20.00 grams
Yeast extract – 2.000 grams
Mannitol – 20.00 grams
Sodium pyruvate – 2.000 grams
Sodium chloride (NaCl) – 1.000 grams
Magnesium sulfate – 0.010 grams
Sodium deoxycholate – 0.500 grams
Neutral Red – 0.030 grams
Crystal Violet – 0.001 grams
CTN selective supplement (FD034) – 2 Vials
Agar – 12.50 grams
Final pH 7.4 ±0.2 at 25°C
(Reference: https://www.himedialabs.com/media/TD/M843.pdf)
Components per 1 Vial of CTN Selective Supplement (FD034)
Cefsulodin – 7.500 mg
Irgasan (Triclosan) – 2.000 mg
Novobiocin – 1.250 mg
(Reference: https://www.himedialabs.com/media/TD/FD034.pdf)
Preparation of CIN Agar Medium (HiMedia)
- Suspend 58.04 grams of the Yersinia Agar Base medium (or the required amount of each component listed above) in 1000 mL of distilled water in a suitable conical flask or glass bottle.
- Dissolve well using a magnetic stirrer or shaking manually.
- Heat to boiling so that every component including agar dissolves completely in the water.
- Autoclave for 15 minutes at 121°C and 15 lbs pressure to sterilize the medium.
- Let the medium cool for about 45°C.
- Add 2 mL of sterile distilled water and 1 mL of ethanol in each vial of CTN Selective Supplement (FD034) and mix the components by gently shaking the vial. Prepare such 2 vials.
- When the basal medium is cooled to around 45°C, mix the 2 prepared vials of CTN Selective Supplement in the basal medium.
- Mix well with gentle shaking. This will form the workable CIN Agar medium.
- Dispense the required volume of the CIN agar medium in a petri plate or test tube and let it solidify completely before using.
Self-life/Storage Condition
- At 2 to 8°C and in sealed condition, the prepared medium can be stored for about 3 to 8 weeks. The unprepared media components can be stored at 10 to 30°C in a sealed container till the labeled expiration date.
Quality Control and Colony Characters on CIN Agar
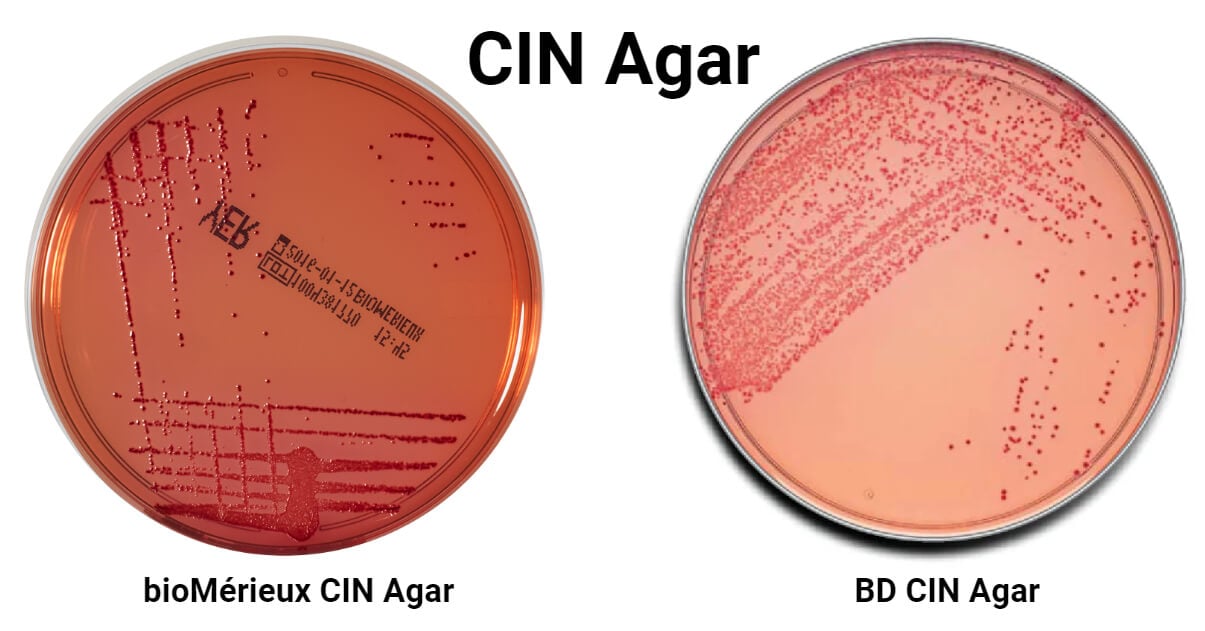
- Unprepared medium powder with neural red indicator will appear yellow to pink colored powder; whereas, prepared and solidified medium in petri plate appears orange-red/pink colored.
- The pH of the medium must be 7.4 ±0.2 at 25°C.
- Cultural characters of some bacteria after 24 to 48 hours of incubation at 22 to 32°C:
| Organism | Appearance |
| Escherichia coli ATCC 25922 | No Growth/Partial Inhibition |
| Pseudomonas aeruginosa ATCC 27853 | No Growth/Partial Inhibition |
| Proteus mirabilis ATCC 25933 | No Growth/Partial Inhibition |
| Salmonella typhimurium ATCC 14028 | No Growth/Partial Inhibition |
| Enterococcus faecalis ATCC 29212 | No Growth |
| Staphylococcus aureus ATCC 25923 | No Growth |
| Yersinia enterocolitica ATCC 27729 | Positive Growth, Bull’s-eye colonies- dark red/pink center with a translucent border |
Precautions
- Do not add CTN Selective Supplement before autoclaving or when the medium is above 50°C or below 40°C.
- Use sterile distilled water to hydrate the CTN selective supplement vial.
- Use the appropriate amount of CTN Selective Supplement otherwise other bacteria can grow.
Limitations of CIN Agar
- Other Yersinia species like Y. pseudotuberculosis, Yersinia frederiksenii, Y. kristensenii, Y. intermedia, etc. can also grow and produce similar colonies.
- Aeromonas, Serratia liquefaciens, Citrobacter freundi, and Enterobacter agglomerans grow and shows similar colonial characteristics.
- The medium only provides presumptive identification. Y. enterocolitica must be confirmed using biochemical or genetic analysis.
Applications
- Isolation and identification of Yersinia enterocolitica strains from clinical (fecal) samples and food samples.
Safety and Disposal
- Dispose of the used medium after autoclaving or incineration.
References
- Schiemann DA. Synthesis of a selective agar medium for Yersinia enterocolitica. Can J Microbiol. 1979 Nov;25(11):1298-1304. doi: 10.1139/m79-205. PMID: 540256.
- Sarovich DS, Colman RE, Price EP, Chung WK, Lee J, Schupp JM, Cobble KR, Busch JD, Alexander J, Keim P, Wagner DM. Selective isolation of Yersinia pestis from plague-infected fleas. J Microbiol Methods. 2010 Jul;82(1):95-7. doi: 10.1016/j.mimet.2010.03.019. Epub 2010 Apr 10. PMID: 20385178; PMCID: PMC2903439.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1
- (1995). Cefsulodin Irgasan Novobiocin (CIN) agar. Progress in Industrial Microbiology, 34, 275-277. https://doi.org/10.1016/S0079-6352(05)80027-X
- https://www.himedialabs.com/media/TD/M843.pdf
- https://www.himedialabs.com/media/TD/FD034.pdf
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/122/409/95760dat.pdf
- https://www.dalynn.com/dyn/ck_assets/files/tech/PY47.pdf
- https://assets.fishersci.com/TFS-Assets/LSG/manuals/IFU1998.pdf
- https://www.bioz.com/result/cin%20agar/product/HiMedia%20Laboratories
- https://www.neogen.com/globalassets/pim/assets/original/10033/official_ncm0182_yersinia-selective-agar-schiemanns-cin-agar_technical-specifications_en-us.pdf
- http://emp.com.ge/culture-media/cin-agar/

Hi Prashant
I am dr Madara Premaratne. A microibiologist .Just went through your above description .I am bit confused with the comment written here as Yersinia spp as Lactose fermenters .Which I believe not
Hi Madara,
Thanks for the correction. It should have been Mannitol instead of Lactose. The page have been updated. 🙂