• Chemical reactions involve the transformation of reactants into new substances, known as products, through the breaking and formation of chemical bonds. • Chemical reactions are symbolically represented using chemical equations, showcasing the reactants on the left, an arrow representing the reaction, and the products on the right. • Chemical reactions are fundamental, driving crucial biological processes like metabolism, respiration, and photosynthesis, which are essential for life and ecosystem sustainability. • In industrial settings, chemical reactions are at the heart of producing various products, including pharmaceuticals, fuels, plastics, and materials. They are vital for technological advancements and innovations. • Chemical reactions are ubiquitous, occurring everywhere, from the cellular level in organisms to global industrial processes.
A chemical reaction is a fundamental process in chemistry where substances, known as reactants, transform to form new substances, known as products.
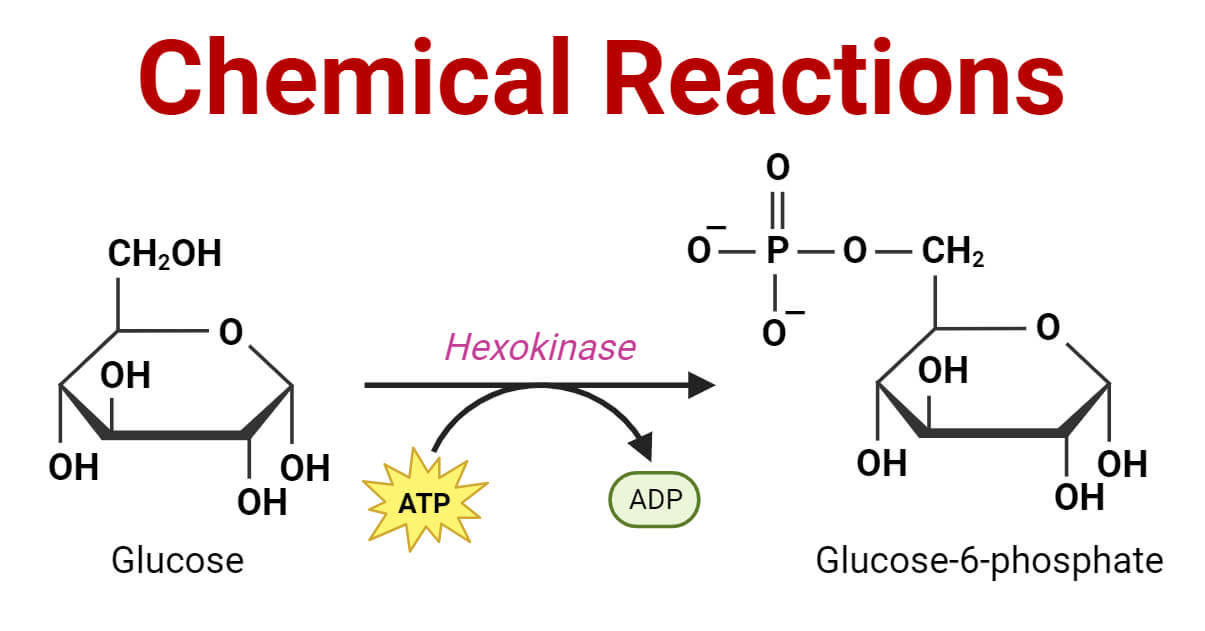
In simpler terms, it’s like rearranging the building blocks (atoms and molecules) to create something new. During a chemical reaction, bonds between atoms are broken and reformed, resulting in a change in the composition and properties of the substances involved.
Interesting Science Videos
Role of Chemical Reactions in the Natural World
Chemical reactions are omnipresent and vital in both the natural world and industrial settings. In nature, chemical reactions drive various biological processes essential for life, such as metabolism, respiration, and photosynthesis.
For instance, photosynthesis in plants is a complex chemical reaction that converts light energy into chemical energy, producing oxygen and sugars as products.
Fundamental Concepts of Chemical Reactions
Atoms are the basic building blocks of matter, each with a nucleus containing protons, neutrons, and electrons orbiting around the nucleus.
When atoms chemically bond, they form molecules. Molecules can be elements (made of identical atoms) or compounds (combinations of different atoms). Compounds have unique properties distinct from their constituent elements.
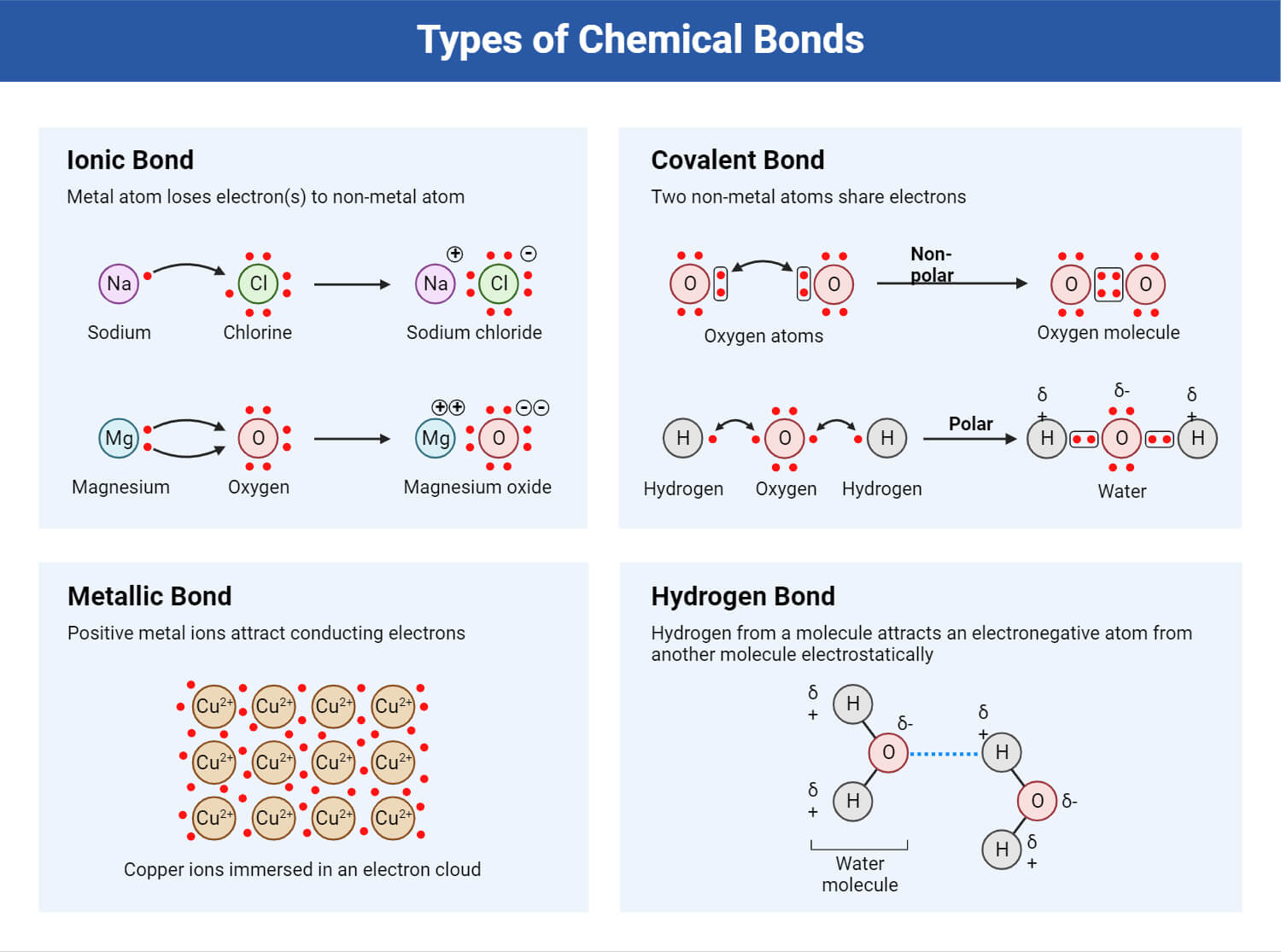
Understanding Chemical Bonds: Covalent, Ionic, and Metallic Bonds
Covalent Bonds: Formed by sharing electrons between atoms, creating a strong bond. This is common in molecules where non-metal atoms combine, like in water (H₂O).
Ionic Bonds: Involve the transfer of electrons between a metal and a non-metal, creating charged ions that attract each other. Common in compounds like table salt (NaCl).
Metallic Bonds: Found in metals, where electrons are shared collectively among a lattice of atoms. This creates unique properties such as conductivity and malleability.
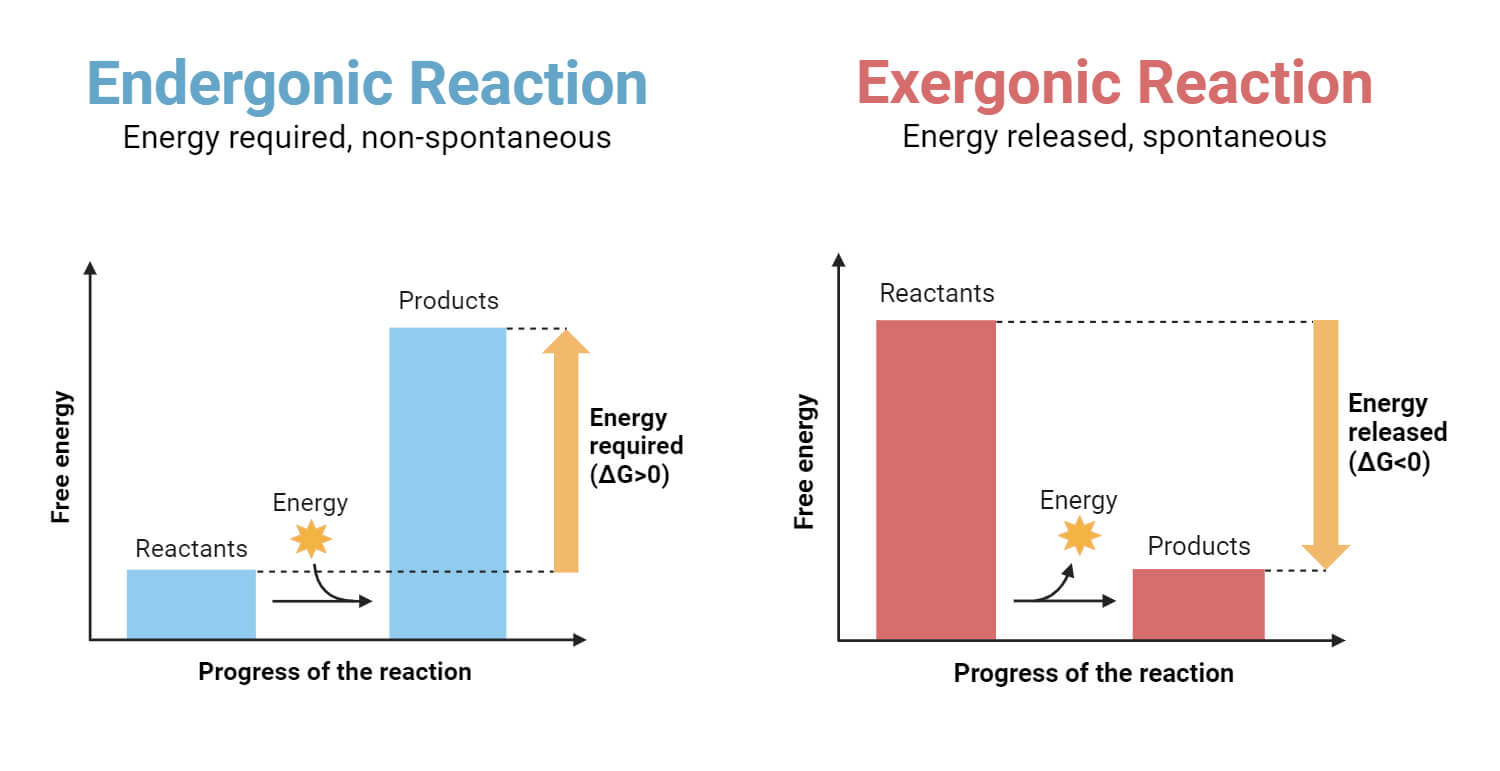
Energy in Chemical Reactions: Endothermic and Exothermic Reactions
Endothermic Reactions: Absorb energy from the surroundings, usually in the form of heat. During these reactions, the products have higher energy than the reactants.
Exothermic Reactions: Release energy to the surroundings, usually in the form of heat or light. The products in these reactions have lower energy than the reactants.
Types of Chemical Reactions
Combination Reactions
Combination reactions occur when two or more substances combine to form a new compound. This could be elements combining to form a compound, like hydrogen and oxygen forming water (2H₂ + O₂ → 2H₂O).
Decomposition Reactions
Decomposition reactions involve the breakdown of a compound into simpler substances. For instance, when water breaks down into hydrogen and oxygen (2H₂O → 2H₂ + O₂).
Displacement Reactions (Single and Double)
Single Displacement Reactions: Involve an element replacing another element in a compound, resulting in a new compound and a free element. For example, when zinc displaces copper in copper sulfate (Zn + CuSO₄ → ZnSO₄ + Cu).
Double Displacement Reactions
It involves an exchange of ions between two compounds, resulting in the formation of two new compounds. An example is the reaction between sodium chloride and silver nitrate (NaCl + AgNO₃ → AgCl + NaNO₃).
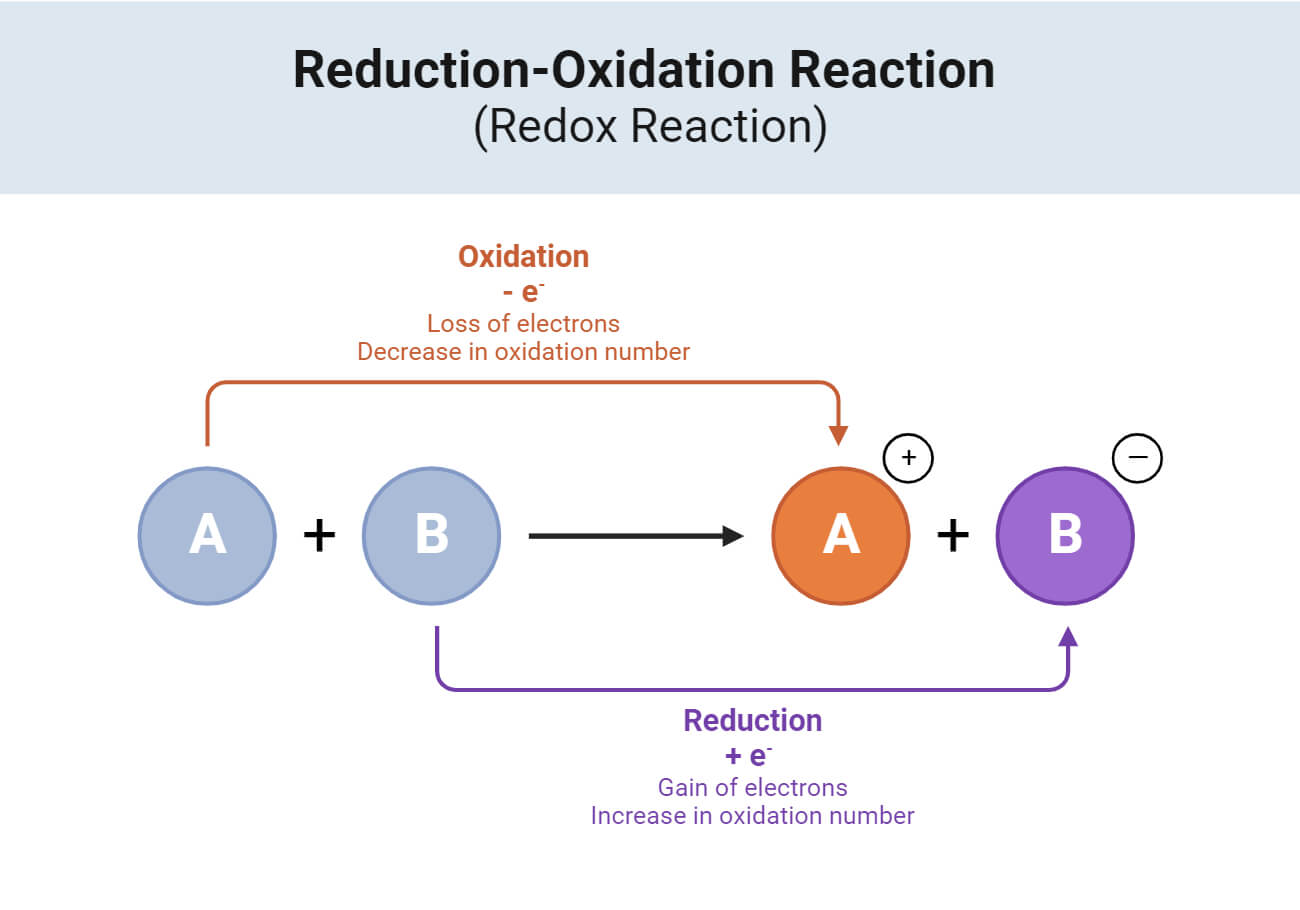
Redox Reactions (Oxidation and Reduction)
Oxidation: Involves the loss of electrons by a substance, often increasing in oxidation state. For instance, when iron rusts (4Fe + 3O₂ → 2Fe₂O₃).
Reduction
It involves the gain of electrons by a substance, often resulting in a decrease in oxidation state. For example, during the reaction of copper(II) oxide with hydrogen (CuO + H₂ → Cu + H₂O).
Balancing Chemical Equations
A balanced chemical equation represents a chemical reaction, illustrating the exact ratio of reactants that combine to form products. It maintains the law of conservation of mass, ensuring that the total mass of elements before and after the reaction remains constant.
Understanding the Law of Conservation of Mass
The law of conservation of mass states that mass is conserved in a chemical reaction; the total mass of the reactants equals the total mass of the products. This fundamental principle underpins the process of balancing chemical equations.
Step-by-Step Method for Balancing Chemical Equations
Balancing chemical equations involves adjusting coefficients to achieve an equal number of atoms of each element on both sides. It often follows a systematic approach of starting with complex molecules and incrementally adjusting coefficients.
Factors Influencing Chemical Reactions
Temperature and Its Effect on Reaction Rate
Temperature significantly influences reaction rates. Higher temperatures generally accelerate reactions by increasing the kinetic energy of particles, leading to more frequent and energetic collisions.
Concentration and Pressure
Concentration (for solutions) and pressure (for gases) affect reaction rates. Higher concentrations or pressures result in more collisions between molecules, thus increasing the rate of reaction.
Catalysts and Inhibitors
Catalysts speed up chemical reactions by providing an alternative reaction pathway with lower activation energy, while inhibitors decrease the reaction rate by interfering with the reaction process.
Chemical Reaction Rates
Reaction rate refers to the speed at which a chemical reaction takes place, usually expressed as the change in concentration of reactants or products per unit time.
Factors Affecting Reaction Rates: Temperature, Concentration, Catalysts, Surface Area
Factors affecting reaction rates include temperature (higher temperature, faster rate), concentration (higher concentration, faster rate), catalysts (speed up the reaction), and surface area (larger surface area, faster rate).
Chemical Equilibrium
Chemical equilibrium is a state in a reversible reaction where the concentrations of reactants and products remain constant over time. It is a dynamic balance where the forward and reverse reactions occur at equal rates.
Equilibrium Constant (K) and Its Significance
The equilibrium constant (K) quantifies the relationship between concentrations of reactants and products at equilibrium. It indicates the extent of the reaction and predicts the direction the reaction will proceed.
Le Chatelier’s Principle and Its Application to Chemical Equilibrium
Le Chatelier’s Principle predicts how a system at equilibrium responds to changes in temperature, pressure, or concentration. The system adjusts to counteract the change and re-establish equilibrium.
Conclusion
From balancing equations to grasping factors affecting rates and equilibrium, is pivotal in chemical sciences. It enables precise prediction and control of reactions, playing a key role in various scientific and industrial applications.
A thorough understanding of these concepts enriches our ability to navigate and manipulate the chemical transformations shaping our world.
Reference
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Chemical_Reactions/Chemical_Reactions_Examples/Chemical_Reactions_Overview
- https://chemistrytalk.org/types-of-chemical-reactions/
- https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch3/equations.html
- https://energyeducation.ca/encyclopedia/Chemical_reaction
- https://courses.lumenlearning.com/wm-biology1/chapter/reading-chemical-reactions-and-molecules-2/
- https://open.oregonstate.education/aandp/chapter/2-3-chemical-reactions/
- https://www.ck12.org/chemistry/chemical-reaction/
