Carbohydrate Fermentation Test is the biochemical test used to assess the ability of bacteria to ferment a specific carbohydrate and to differentiate bacteria based on their carbohydrate fermentation pattern and identify them.
Not all bacterial groups have the same nutritional requirement and biochemical properties. Different bacteria might have different enzyme systems making them different in substrate-utilizing ability. A similar pattern is seen in carbohydrates (sugars) utilizing the ability of bacteria – some can use one type of sugar while others can’t use it at all. Also, the mode of metabolizing the same sugar and the end products of metabolism may vary in the different bacterial groups – some can use fermentative mode while some can oxidative mode of metabolism, some produce acids as end product while some produce alcohol, some may release gas while some may not.
Carbohydrates, simply called sugars, are the polyhydroxy aldehydes or ketones that are composed of carbon (C), hydrogen (H), and oxygen (O) atoms combined in an empirical combination of Cx(H2O)y. There are numerous types of carbohydrates classified on different bases. However, in microbiology and carbohydrate fermentation tests, glucose, lactose, sucrose, maltose, mannitol, galactose, starch, rhamnose, aesculin, salicin, adonitol, dulcitol, sorbitol, cellobiose, xylose, mannose, trehalose, inositol, raffinose, melibiose, and cellulose are commonly used as substrate.
During the fermentation process, the carbohydrate molecules are anaerobically catabolized into organic acids. Thus produced acid decreases the pH of the medium and compels the pH indicator to change its color.
But not all bacteria have the ability to ferment above mentioned carbohydrates. Some bacterial species can ferment some of these carbohydrates while some might not ferment them. This ability of bacteria to ferment a specific carbohydrate can be used to classify bacteria into different groups and aid in the bacterial identification process. The test used to determine the carbohydrate fermenting pattern and ability of a microorganism is called the carbohydrate fermentation test.
Interesting Science Videos
Objectives of Carbohydrate Fermentation Test
- To assess the ability of bacteria to ferment a specific carbohydrate.
- To differentiate bacteria based on their carbohydrate fermentation pattern and identify them.
Principle of Carbohydrate Fermentation Test
Different bacteria have different abilities to ferment specific carbohydrates – some can ferment one type of carbohydrate while others can’t ferment the same sugar. While fermenting carbohydrates, organic acids are produced as end products. These produced acids will decrease the pH of the medium. This decrease in pH causes the pH indicator present in the medium to change color. (The color change depends on the type of pH indicator used in the test.)
If the bacteria release gases during carbohydrate fermentation, they will get trapped as an air bubble in the inverted Durham tube/fermentation tube submerged inside the fermentation broth.
This color change indicates the occurrence of fermentation and the formation of an air bubble in Durham’s tube indicates the release of gas and hence, confirms that the test bacteria were able to use the specific carbohydrate used in the test medium.
Requirement for Carbohydrate Fermentation Test
a. Culture Media
Carbohydrate Broth can be used for performing this test. The composition of the medium can be modified according to the test organism and its source or components available in the lab. In this article, we will be using a composition based on “Carbohydrate Consumption Broth Base” by HiMedia (M124) with a certain change in pH indicator and will add specific carbohydrates.
Composition of Carbohydrate Broth Base per 990 mL
Proteose Peptone- 10.00 grams
HM Peptone B (Beef extract)- 1.00 grams
Sodium Chloride- 5.00 grams
Phenol Red- 0.018 grams
(Alternatively, Bromocresol purple 0.100 grams can be used)
Preparation of Carbohydrate Broth
- Measure the appropriate amount of media components and dissolve them in 990 mL of distilled water.
- Add 10 grams of specific carbohydrates for the test and dissolve by shaking.
- Heat to boiling if needed for the complete dissolution of components.
- Dispense about 5 to 7 mL (or the volume required to submerge Durham’s tube) of the broth to test tubes.
- Submerge a Durham tube in each tube completely and make sure there is no air bubble inside the submerged Durham tube.
- Loosely put on the cap or cotton plug and autoclave the tubes for 15 minutes at 1210C and 15 lbs pressure.
b. Carbohydrates
Glucose, lactose, sucrose, maltose, mannitol, galactose, starch, rhamnose, aesculin, salicin, adonitol, dulcitol, sorbitol, cellobiose, xylose, mannose, trehalose, inositol, raffinose, melibiose, and cellulose are carbohydrates commonly used as substrate in the carbohydrate fermentation test.
c. Reagents
pH indicators are required to detect the production of acid in the medium. Different pH indicators can be used like:
- Andrade’s Indicator: light pink at about neutral pH range (7.1 to 7.2), turns dark pink to red at acidic pH (at around or below 5.0 ), and turns yellow at high alkaline pH (about 12 to 14)
- Phenol Red: Reddish orange at neutral (about 7.4 pH), turns yellow at acidic pH (at and below 6.8 pH), and turns pink-red at alkaline pH (at or above 8.4 pH)
- Bromocresol Purple: Deep purple at about neutral (about 7.4 pH), turns yellow at acidic pH (at and below 5.2 pH), and turns purple at alkaline pH (at and above 6.8 pH)
- Bromothymol Blue: Green at neutral pH (about 7.0 pH), turns yellow at acidic pH (at and below 6.0 pH), and turns Prussian blue at alkaline pH (at and above 8.4 pH)
Based on the range of pH for the color change, toxicity, ease to notice the change, and availability, phenol red is the most preferred pH indicator.
d. Equipment
| Test-tubes Inoculating Loop | Weighing Machine Autoclave & Incubator | Bunsen burner | Durham’s tubes |
PPE and other general laboratory materials
e. Test Organisms (Sample Bacteria)
(Carbohydrate fermentation test is also used for the differentiation of yeasts species like Candida spp. and Saccharomyces spp.; hence, the test organisms may be yeasts.)
Procedure of Carbohydrate Fermentation Test
- Using a sterile inoculating loop, pick up a well-isolated colony from a fresh culture (18 to 24 hours old culture) of sample bacteria and inoculate the broth.
- Incubate the tubes at 35±2°C for 18 to 24 hours.
- Observe for a color change of the broth and trapped air bubbles in Durham’s tube.
- If no color change or air bubble is seen, re-incubate the tubes for the next 24 hours and observe for color change and air bubbles. Some may need incubation of 5 days or more.
Result Interpretation of Carbohydrate Fermentation Test
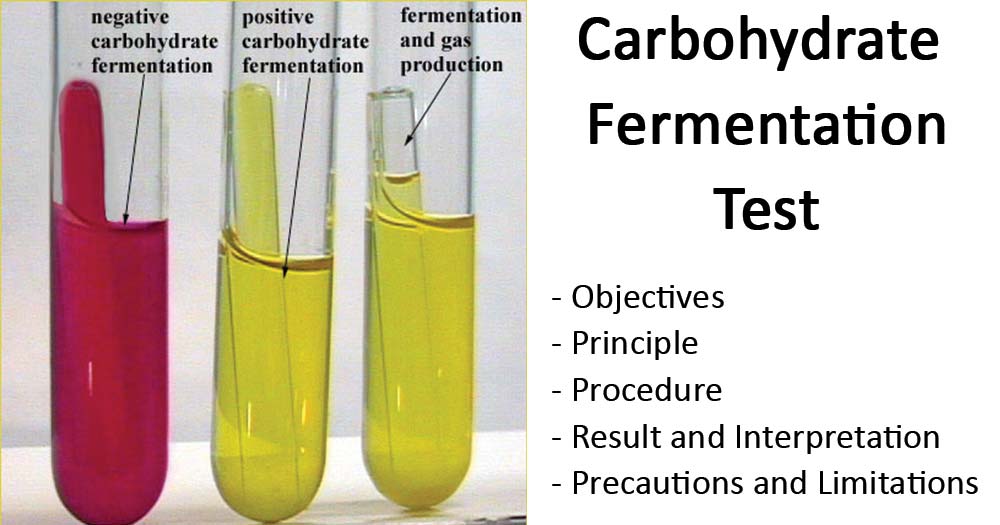
- Positive fermentation is denoted by the color change of the media from reddish-orange to yellow.
- Negative fermentation is denoted by no color change of the medium (remains reddish-orange).
(If another pH indicator is used, read the color change accordingly.)
- Gas production is indicated by the formation of an air bubble in Durham’s tube.
| Observation | Result | Interpretation |
| The medium changes to yellow color | Acid production | Organism ferments the given carbohydrate and produces organic acids thereby reducing the pH of the medium into acidic conditions. |
| The medium changes to yellow color and production of gas formation in the Durham tube | Acid and Gas production | Organism ferments the given Carbohydrate and produces organic acids and gas. Gas production is detected by the presence of small bubbles in the inverted Durham tubes. |
| No change in color (retains red color) | Absence of fermentation | The organism cannot utilize the carbohydrate but the organism continues to grow in the medium using other energy sources in the medium. |
Fermentation Pattern of Some Common Bacteria
| Name of Bacteria | Fermentation Result of | Gas Production | ||||||||
| Glucose | Lactose | Sucrose | Maltose | Arabinose | Cellobiose | Mannitol | Sorbitol | Xylose | ||
| E. coli | + ve | + ve | V | + ve | + ve | -ve | + ve | + ve | + ve | + ve |
| K. pneumoniae | + ve | + ve | + ve | + ve | + ve | + ve | + ve | + ve | + ve | + ve |
| K. oxytoca | + ve | + ve | + ve | + ve | + ve | + ve | + ve | + ve | + ve | V |
| Proteus mirabilis | + ve | -ve | -ve | -ve | -ve | -ve | -ve | -ve | + ve | + ve |
| Pseudomonas aeruginosa | + ve | -ve | -ve | -ve | -ve | -ve | + ve | -ve | -ve | -ve |
| Salmonella Typhi | + ve | -ve | -ve | + ve | -ve | -ve | + ve | -ve | + ve | -ve |
| Shigella flexneri | + ve | -ve | -ve | V | V | -ve | + ve | V | -ve | + ve |
| Shigella dysentery | + ve | -ve | -ve | V | V | -ve | -ve | V | -ve | – ve |
Precaution
- Be sure there is no air bubble in the inverted Durham tube.
- While inoculation, avoid touching (inoculating over) the Durham tube. Swirl the media to ensure proper distribution of the sample in the broth.
- Some may need prolonged incubation, up to 5 days or even more, so don’t report negative after 24 hours.
Applications of Carbohydrate Fermentation Test
- Differentiation of Gram-negative bacilli
- Presumptive identification of bacteria
- Differentiation of Candida spp.
Limitations of Carbohydrate Fermentation Test
- It is not a confirmatory test; hence, requires test results of other biochemical tests for complete identification.
- Diverse types of carbohydrates and indicators are available. Lots of media, each containing a specific carbohydrate is to be made.
- If the result is read too late, oxidative deamination may occur, resulting change of medium to alkaline pH and causing a false negative result.
- May require more than 24 hours for a positive reaction. False results can be obtained if the result is read too early.
- Even minor contamination can result in a false positive result.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- A. Hassan, Baydaa. (2018). Carbohydrate fermentation test & starch hydrolysis test. 10.13140/RG.2.2.21943.57762.
- Carbohydrate Fermentation Protocol (asm.org)
- Carbohydrate Fermentation Test: Uses, Principle, Procedure, Results (microbeonline.com)
- Raffinose fermentation test – Virtual Microbiology Lab Simulator Software (vumicro.com)
- 7.1: Introduction to Biochemical Tests Part I – Biology LibreTexts
- Carbohydrate Fermentation Test on Bacteria to find-out their Ability to Ferment Carbohydrates (yourarticlelibrary.com)
- Sugar Fermentation Test (Carbohydrate Fermentation Test) – BiochemGems
- Carbohydrate Fermentation Test: Principle, result, & Uses (researchtweet.com)
- 3.1: Carbohydrate Fermentation – Biology LibreTexts
- Fermentation Test – Principle, Procedure, Uses and Interpretation (microbiologyinfo.com)
- Microbiology – 007 – Carbohydrate Fermentation Test | Microbiology Undergraduate Program (iastate.edu)

Can you test for other carbohydrates if the organism cannot ferment glucose? Why or why not?
Why is liquid medium preferred over solid
Composite medium for identification of bacteria