Carbapenems are members of the β-Lactam antibiotic class. Molecularly defining, carbapenems are antibiotics having β– lactam ring with sulfur at the C-1 position and a double bond between C-2 and C-3 of the ring with the side chains arranged in the Trans- position.
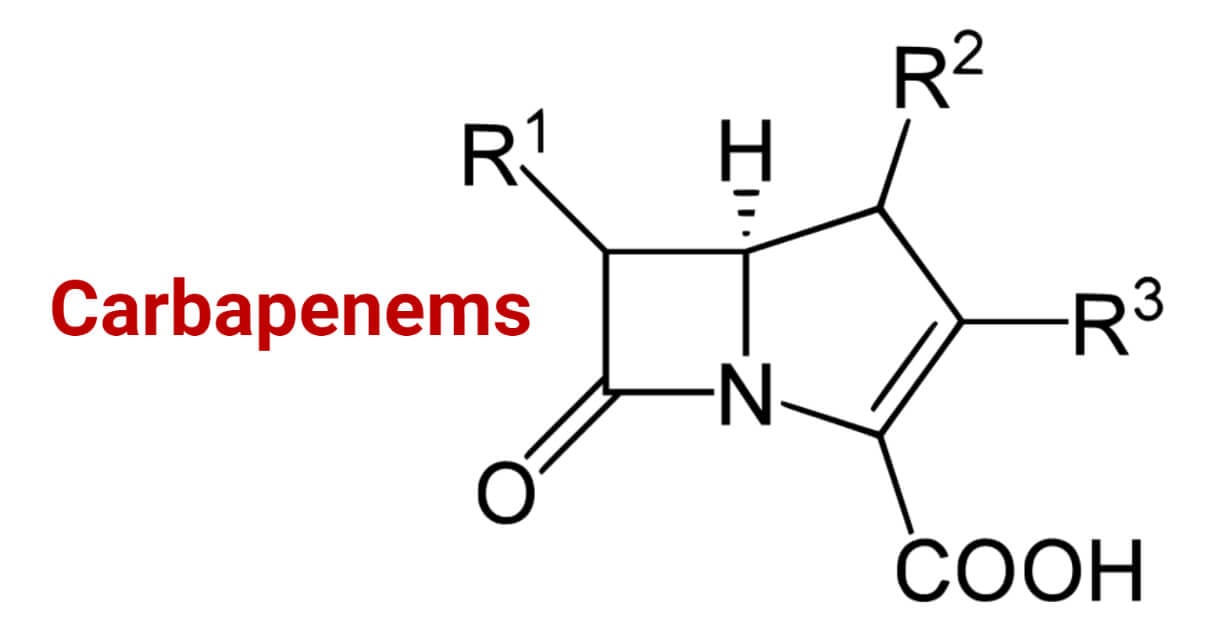
- This unique arrangement of the side chains in the trans position, unlike in the cis position like in other β-lactams, makes them resistant to several β– lactamases like Extended Spectrum β-lactamases (ESBLs), Penicillinases, Cephalosporinases, AmpC β– lactamases, etc. However, they are susceptible to carbapenemases and Metallo-beta-lactamases.
- They are the most potent antibiotic agents and are widely used for treating infections associated with multi-drug-resistant bacteria. They show a broader spectrum of activity and are less likely to be resisted quickly than other members of the β- Lactam antibiotic class. They are reserved antibiotics and are used only when other available antibiotics fail to cure the infection.
- They are on the watch group of essential medicines list by the World Health Organization (WHO) to treat severe Gram-negative bacterial infections.
- They are called “the antibiotics of last resort” or “the last line agents”
Interesting Science Videos
Carbapenems History
- During the search for β– lactamases inhibitors, Olivanic Acids were discovered in 1976. These were the first compound discovered to have the carbapenem backbone but were not used as antibiotics due to very low stability and poor penetration power. In a further study, in 1976, ‘Thienamycin’ was found to be produced by Streptomyces cattleya. This was a stable compound and showed good effectiveness against bacteria. It was the first naturally obtained carbapenem antibiotic.
- It was put in the clinical trial as named “Thienpenem” antibiotic. Unfortunately, it was found unstable in an aqueous solution, sensitive to bases above pH 8.0, and reactive to nucleophiles, therefore, was not used as a commercially produced antibiotic.
- Thienpenem is the parent carbapenem antibiotic and model for all other carbapenems currently available.
Mechanism of Action of Carbapenems
- As like other β- Lactam antibiotics, carbapenems affect the bacterial cell wall and prevent bacterial cell wall synthesis resulting in bacterial cell lysis. They show bactericidal properties against both Gram-positive and Gram-negative bacteria.
- Carbapenems bind to the penicillin-binding proteins (PBPs) (also called DD-transpeptidases) and inactivate them. This prevents the final transpeptidation step during the bacterial cell wall synthesis (peptidoglycan synthesis) resulting in no elongation and crosslinking of peptidoglycan in the bacterial cell wall. The bacteria can’t develop their cell wall and ultimately die.
- Similarly, the prevention of transpeptidation and crosslinking results in the accumulation of nascent peptidoglycans. They trigger the production of autolytic hydrolases which digest the existing peptidoglycan layer without the formation of a new one and further enhance the bactericidal effect.
Types of Carbapenems
Since the discovery of the 1st carbapenem thienpenem, several carbapenems with different spectra, pharmacology, and safety profile are discovered and synthesized. Some are already in clinical use while some are still under study.
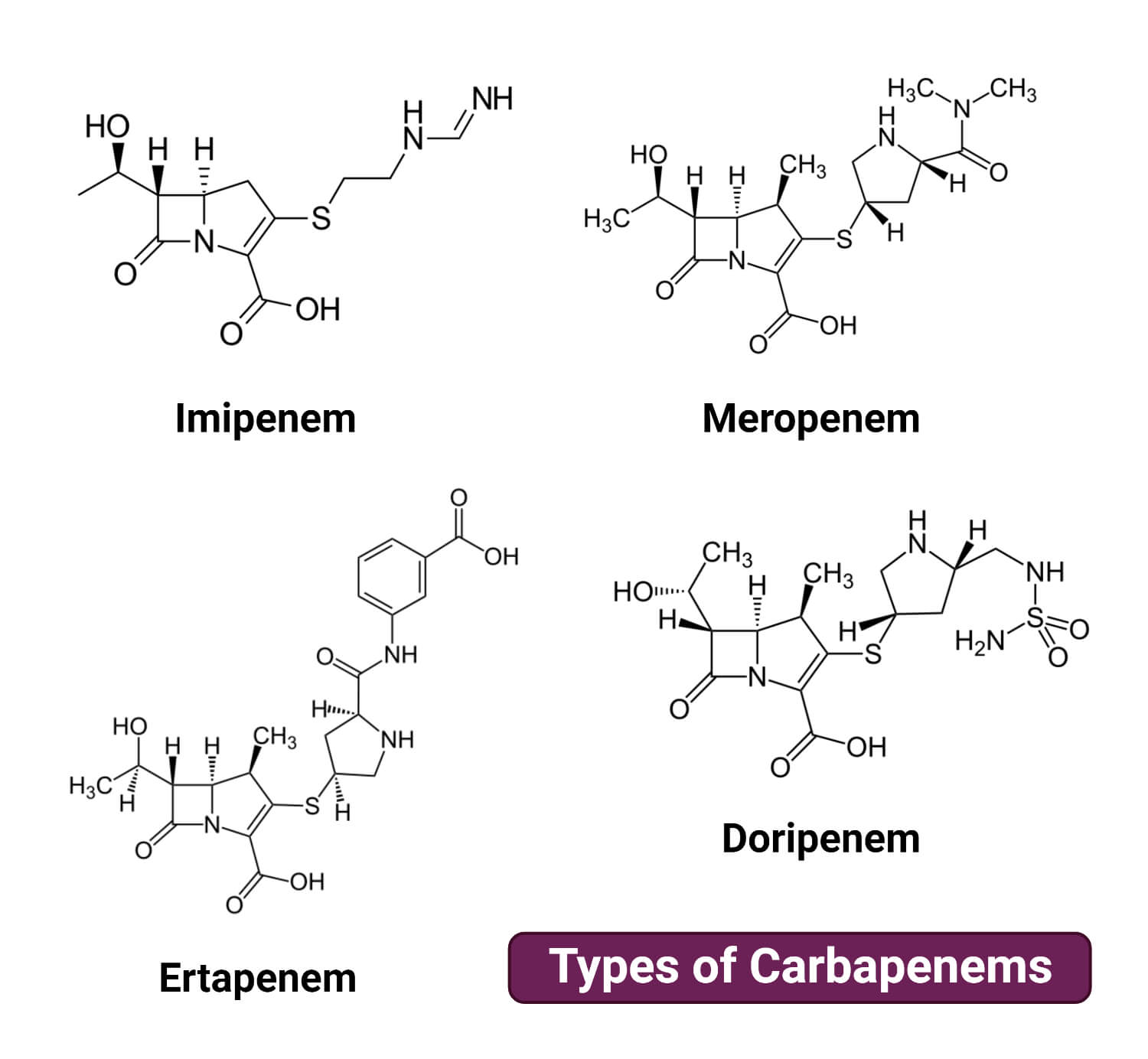
The most commonly used carbapenems are:
Imipenem
It is the N-formimidoyl derivative of Thienamycin, also called as N-Formimidoyl thienamycin. It was It is the first carbapenem antibiotic available in the market for treatment since 1985. It was originally called MK0787 and later marketed under the name ‘imipenem’.
Molecular formula: C12H17N3O4S
It can be deactivated by dehydropeptidases I (DHP-I) at the human renal brush border; hence it is always co-administrated with inhibitors like cilastatin or betamipron in a ratio of 1:1 by weight. Despite this limitation and its side effects, imipenem is still one of the widely used carbapenem antibiotics. It is mainly used in infections of P. aeruginosa, Enterococcus, septicemia, bone and joint infections, UTIs and intra-abdominal infections by Gram-negative bacteria, and pneumonia.
Meropenem
It is another derivative of Thienamycin. It contains β –methyl in its side chain at the C1 position, unlike imipenem which contains H at the C1 position. This difference makes meropenem more stable and resistant to the effect of DHP-I.
It has lower activity against Gram-positive bacteria and higher activity against Gram-negative bacteria than imipenem. Hence, meropenem is a more potent carbapenem with a wider spectrum than imipenem.
It is safe and well tolerated and is listed in the WHO’s List of Essential Medicines. It has proven itself a very important medicine in treating infections by MDR Gram-negative bacteria since its approval and availability in 1995. It is widely used in the treatment of meningitis and CNS infections, skin infections, sepsis and bacteremia, and intra-abdominal infections.
Molecular formula: C17H25N3O5S
Ertapenem
It is another approved carbapenem with completely different properties than imipenem and meropenem. It was first approved in 2001 and still, it remains as the least prescribed carbapenem. It is generally used in treating Gastrointestinal infections, lung (pleural) infections, female reproductive tract infections, and UTIs. Although it shows higher activity against Gram-negative bacteria than imipenem, it is not effective against Pseudomonas spp., Acinetobacter spp., and Enterococcus spp.
Molecular formula: C22H25N3O7S
Doripenem
It is a newer carbapenem antibiotic that was approved only in 2007 and still, and it is under study and its availability is limited in comparison to other above-mentioned carbapenems. It is generally used against infections of Pseudomonas spp., severe bacterial gastrointestinal infections, nosocomial pneumonia, pyelonephritis, and complicated UTIs. It has higher activity against both Gram-positive and Gram-negative bacteria than imipenem and meropenem.
Molecular formula: C15H24N4O6S2
Other carbapenems that are under several stages of study and trials are:
- Tebipenem: It is the first oral carbapenem. It is currently approved in 2009 and is available only in Japan. Recently in July 2022, FDA approved its use for review under the phase III clinical trial stage in the USA in case of complicated UTIs.
- Biapenem
- Tomopenem
- Panipenem
- Sanfetrinem and other trinem carbapenems
- Anti-MRSA Carbapenems (15i and CP5484)
Carbapenem Administrative Route and Recommended Dosage
| Carbapenem Antibiotics | Administrative Route | Dosage (for adult) |
| Imipenem | Intravenous route (IV route) (20 to 60 minutes IV infusion) | 250 to 1000 mg (based on patients age, weight, and renal condition) |
| Meropenem | Intravenous route (IV route) (15 to 30 minutes IV infusion) | 50 to 2000 mg per 8 hours (based on patients age, weight, site of infection, and renal condition) |
| Ertapenem | Intramuscular (IM) or Intravenous route (IV route) | Up to 1000 mg per day/once daily for an adult15 mg/kg or a maximum of up to 1000 mg for pediatric (based on age, site of infection, and renal and hepatic condition) |
| Doripenem | Intravenous route (IV route) (60 minutes IV infusion) | 250 to 500 mg every 8 hours max up to 14 days (based on patients age, weight, site of infection, and renal condition) |
Applications of Carbapenems
Carbapenems have a broad spectrum of activity and are used to treat severe infections, mostly those caused by multi-drug resistant species (MDR Enterobacteriaceae, A. baumannii, Pseudomonas spp., Streptococcus spp., Mycobacterium tuberculosis complex, Haemophilus influenzae, MRSA, Salmonella serovars, etc.). It is mainly used in the following clinical conditions:
- Complicated Urinary Tract Infections (cUTIs)
- Intra-abdominal Infections
- Pneumonia and Pleural Infections and Severe lower Respiratory Tract Infections (RTIs)
- Endocarditis and bloodstream infections (septicemia and bacteremia)
- Skin and soft tissue infections
- Meningitis
- Bone and joint infections
- As it is the last line of antibiotics, it is recommended to administer only when other antibiotics fail to show any activity.
Risks and Limitations with Carbapenem Administration
- Patients with an allergic reaction to β– lactams and patients taking valproic acid for seizure must not use carbapenems
- Patients with neural and hepatic disorders must be reported and dosage must be considered accordingly
- Might show adverse side effects like: hypersensitivity, seizures, diarrhea, low platelet count, anemia, leukopenia, brain damage and dysfunction, liver and renal toxicity, pustules and epidermal necrolysis, increase in heart rate and blood pressure.
Some common side effects include: gastrointestinal disorder, headache, pain in the site of administration, rashes and itchiness, insomnia, altered blood pressure, fever, swelling, shortness of breath. - Imipenem must be administered along with cilastatin and panipenem along with betamipron
- Carbapenemases and Metallo-beta-lactamases can hydrolyze them reducing their potency
- Expensive and difficult to find in comparison to other beta-lactam antibiotics
- Available only in form of IV and IM, hence difficult to self-administer and use outside hospital settings.
References
- Nicolau DP. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother. 2008 Jan;9(1):23-37. doi: 10.1517/14656566.9.1.23. PMID: 18076336.
- Thienamycin (chemeurope.com)
- Francis P. Tally, Nilda V. Jacobus and Sherwood L. Gorbach. In Vitro Activity of Thienamycin. https://doi.org/10.1128/AAC.14.3.436
- Papp-Wallace, K. M., Endimiani, A., Taracila, M. A., & Bonomo, R. A. (2011). Carbapenems: Past, Present, and Future. Antimicrobial Agents and Chemotherapy, 55(11), 4943-4960. https://doi.org/10.1128/AAC.00296-11
- National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 104838, Imipenem. Retrieved December 17, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/Imipenem.
- Imipenem Vs Meropenem: The Ultimate Guide | OCTAGONCHEM
- Ertapenem Uses, Side Effects & Warnings – Drugs.com
- Ertapenem dosage and administration – wikidoc
- Doripenem dosage and administration – wikidoc
- Jain A, Utley L, Parr TR, Zabawa T, Pucci MJ. Tebipenem, the first oral carbapenem antibiotic. Expert Rev Anti Infect Ther. 2018 Jul;16(7):513-522. doi: 10.1080/14787210.2018.1496821. Epub 2018 Jul 27. PMID: 30014729.
- Perry CM, Ibbotson T. Biapenem. Drugs. 2002;62(15):2221-34; discussion 2235. doi: 10.2165/00003495-200262150-00005. PMID: 12381221.
- List of carbapenems uses, common brands, and safety information – Desinflamar
- Shah PM. Parenteral carbapenems. Clin Microbiol Infect. 2008 Jan;14 Suppl 1:175-80. doi: 10.1111/j.1469-0691.2007.01868.x. Erratum in: Clin Microbiol Infect. 2008 May;14 Suppl 5:21-4. PMID: 18154543.
- Carbapenems – Bronchitis – AntiinfectiveMeds.com
- Liakopoulos, A., Mevius, D., & Ceccarelli, D. (2016). A Review of SHV Extended-Spectrum β-Lactamases: Neglected Yet Ubiquitous. Frontiers in Microbiology, 7. https://doi.org/10.3389/fmicb.2016.01374
- Christopeit, T., Yang, W., Yang, K., & Leiros, K. S. (2016). The structure of the metallo-β-lactamase VIM-2 in complex with a triazolylthioacetamide inhibitor. Acta Crystallographica. Section F, Structural Biology Communications, 72(Pt 11), 813-819. https://doi.org/10.1107/S2053230X16016113
- Shakil S, Azhar EI, Tabrez S, Kamal MA, Jabir NR, Abuzenadah AM, Damanhouri GA, Alam Q. New Delhi metallo-β-lactamase (NDM-1): an update. J Chemother. 2011 Oct;23(5):263-5. doi: 10.1179/joc.2011.23.5.263. PMID: 22005056.
