A member of a group of short chains fatty acids (SCFA) of four carbon, Butyric Acid is a volatile compound with a formula CH3CH2CH2COOH, which is naturally produced by bacteria in anaerobic conditions.
It is a colorless liquid with an oily texture and unpleasant smell like rancid butter. This compound has proven to be of use in food, chemical, animal feed, and pharmaceutical industries and is an important component of microbial fauna in the mammalian gut. Commercially important for the manufacturing of flavoring agents and in plastics such as cellulose butyrate. Artificial production of butyric acid is mainly carried out by chemical synthesis via butyraldehyde oxidation.
Butyric Acid, also known as Butanoic acid, is a fatty acid of four carbon chains with a carboxylic functional group attached at the end of a chain.
- They are commonly available in esterified forms in plant oils and animal fats.
- In humans, they are present in breast milk, in body odor, and in the colon, produced by the bacterial population by fermenting the carbohydrates and dietary fibers consumed.
- In commercial products, like parmesan cheese and rancid butter, impart an important role in taste and aroma.
Interesting Science Videos
Butyric Acid Chemical and Physical Properties
- Chemical formula – CH3CH2CH2COOH
- Molecular weight – 88.11 g/mol
- Corrosive to tissues and metals
- Colorless and oily textured liquid.
- It tastes like butter.
- Soluble in water, alcohol, propylene glycol, and in most oils.
What is Fermentation?
The process of fermentation has been used as one of the oldest biotechnological tools to produce commercial products like yogurt, wine, beer, cheese, and so much more. Fermentation word is derived from the Latin word ‘fervere,’ meaning boiling, due to the evolution of carbon dioxide.
- In living organisms, fermentation provides an alternate biochemical process of generating energy in the absence of oxygen molecules.
- Microorganisms like yeast, bacteria, and fungi use fermentation in anaerobic conditions to transform sugar into ATP, water, alcohol, lactic acid, and CO2.
- Fermentations are of two types: Lactic acid and Alcoholic fermentation. In Lactic acid fermentation, the substrate for a chemical reaction is ‘Lactose,’ and the specific end product is ‘Lactic acid,’ while in alcoholic fermentation substrate is glucose, and the specific end products are alcohol molecules and carbon dioxide.
- Its uses led to the production of food products like bread, yogurt, cheese, kimchi, and more.
Butyric Acid Fermentation
Butyric acid has several applications in the food and chemical industries. In the food industry, butyric acid is used as a flavor enhancer to get butter-like tastes, and its esters are used to impart aromas and fruit flavors. To overcome the issue of mass production of butanol, food manufacturers prefer fermentation as a useful biotechnological process.
Butyric Acid Fermentation Principle
The general principle for mass production of butyric acid via fermentation includes the glycolysis of glucose molecules by bacterial strain. The glucose is oxidized to pyruvate and further to acetyl-CoA molecule by the action of the enzyme ‘pyruvate ferredoxin oxidoreductase.’ Some acetyl-CoA forms acetic acid, while the rest transforms to acetoacetyl-CoA, which is further reduced to butyryl-CoA and finally converted to butyric acid and ATP with the evolution of carbon dioxide.
End products of this process include butyric acid, 3 molecules of ATP, water, CO2, and a small amount of ethanol.
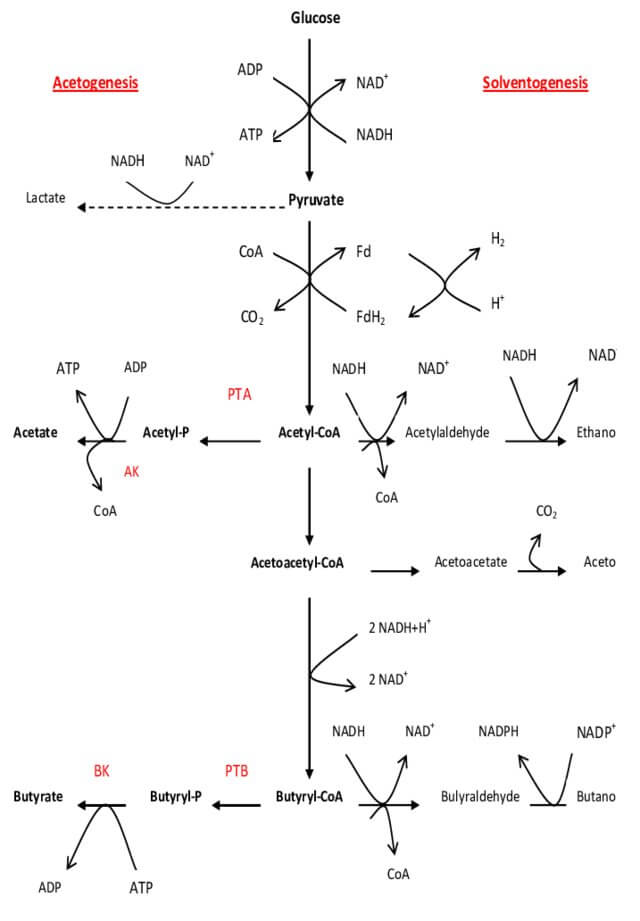
Butyric Acid Fermentation Steps
Microorganisms and Inoculum Preparation
Many bacterial strains, which include species from genera Clostridium, Eubacterium, Butyribacterium, Megasphera, Sarcina, and Fusobacterium, anaerobically produce butyric acid.
- Clostridium species have been widely used for commercial purposes due to the specific characteristics they impart, which include chemo-organotrophy, gram positivity, spore formation, and strict anaerobiosis.
- Media Preparation – This medium contains many chemicals such as ammonium sulfate [(NH4)2SO4], manganese II sulfate (MnSO4), potassium dihydrogen phosphate (KH2PO4), and ferrous sulfate (FeSO4), with yeast extract mixed in distilled water at sterile conditions. Glucose is added at different concentrations as a carbon source.
- Clostridium butyricum is stored at 4 degrees celsius in a medium containing calcium carbonate and medicinal oil to maintain the anaerobic condition.
- Inoculum Preparation – 10ml of sterile medium is poured into the test tube with C. butyricum, incubated for 20 hours at 37 degrees Celsius, and to maintain the pH, CaCO3 (calcium carbonate) is added.
- The end products of the metabolic pathways in Clostridium species include butyrate, butanol, and acetate, with small amounts of lactate and ethanol as byproducts.
Extractive Measure- Batch Fermentation
- This process is performed in a double-walled cylinder with a sample tube and a stirrer and sterilized before the addition of materials.
- The working volume of inoculum is 225 ml (10% v/v) and is prepared in a sterilized medium.
- All the sterilized materials are poured into the cylinder, along with the organic phase, which contains Sucrose (sole carbon source), amines, and some emulsifiers like oleyl alcohol.
- Anaerobiosis is achieved by sparging carbon dioxide gas into the medium containing the inoculum.
- Fermentation is carried out at 37 degrees Celsius, and at regular intervals, samples were taken out for analysis during the whole process.
- Butyric acid is extracted from the organic phase in an aqueous solution, and its concentration is measured by gas chromatography.
Fermentation by Fed-Batch Measure
- All the ingredients and procedures are the same as the batch method of fermentation, except for the addition of Sucrose or Glucose at regular intervals.
- Sterile conditions are to be maintained during the process to avoid the growth of any pathogens.
- The optimum pH maintained is 6.5 for bacterial growth and butyric acid production.
- A comparison of fed-batch and batch fermentation led to the conclusion that the fed-batch method showed enhanced butyric acid production with 36.65% increased butyrate substrate than in batch fermentation.
Limiting Factors in Butyric Acid Fermentation
- High sugar and salt concentrations cause osmotic pressure to the bacterial strain leading to the modification in the phospholipid structure of a cell membrane, causing cell mortality.
- Butyric acid inhibition – a phenomenon occurring due to increased production of acid, affecting the cell membrane integrity and metabolic regulation, which leads to arrested fermentation and low productivity of butyric acid.
- The productivity of butyric acid is affected by a pH-controlled medium for the optimum growth and enzyme activity of bacterial species.
- The use of inappropriate nutrients can lead to decreased productivity of butanoic acid. To avoid situations like this one such supplement is CSL (Corn steep liquor), it is a rich source of amino acids, peptides, degradable proteins, and trace elements.
Butyric Acid Applications
Butyric acid has many industrial applications owing to its exceptional biochemical features.
- As Bioplastic – Used in the production of CAB (cellulose acetate butyrate), which is more resistant towards degradation than cellulose acetate, is applied to coatings, paints, and tools.
- Esters of butyric acids, such as methyl butyrate, impart a pleasant aroma that is used in perfumes, disinfectants, and flavorings.
- As a flavoring agent in cheese, beer, and wine.
- As fishing bait due to its powerful odor, which helps in attracting fish.
Human Health and Butyric Acid
- Circulating butyrate plays a key role in the regulation of immune homeostasis and enhances regulatory T-cells differentiation.
- Gut butyrate helps in the inhibition of pro-inflammatory cytokines and protects the integrity of the intestinal epithelial barrier.
- Butyric acid produced by colonic bacteria helps in the prevention of colon cancer and ulcerative colitis by increasing energy production and inhibiting cell proliferation.
Conclusion
Butyric acid is a volatile short-chain fatty acid (SCFA) compound of 4-carbon as the backbone with a carboxylic group at the 4th carbon atom. In humans, it is found in the gut and in the colon, produced anaerobically by the bacterial strain present there, and helps in the prevention of inflammatory-related diseases. Due to their characteristic features, such as distinct taste, flavor, and pleasant aromas, they are industrially mass-produced by fermentation. Two modes of technique can be applied, which include batch and fed-batch measures. Research has shown fed-batch method is more efficient with increased butyric acid production. Industrial applications consist of the production of bioplastic pigments in paints and coatings; flavoring agents in food, medicines, cheese, and alcohol; esters in perfumes and disinfectants.
References
- Jiang, Ling, et al. “Butyric acid: Applications and recent advances in its bioproduction.” Biotechnology Advances 36.8 (2018): 2101-2117.
- The Future of Butyric Acid in Industry – https://www.hindawi.com/journals/tswj/2012/471417/
- butyric acid – https://www.britannica.com/science/butyric-acid
- Qureshi, Nasib, Siqing Liu, and Badal C. Saha. “Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062.” Fermentation 8.10 (2022): 491.
- Butyric Acid – https://pubchem.ncbi.nlm.nih.gov/compound/Butyric-acid
- Fermentation: Humanity’s Oldest Biotechnological Tool – https://kids.frontiersin.org/articles/10.3389/frym.2021.568656
- Butyric Acid Fermentation- Definition, Principle, Procedure, Uses – https://microbenotes.com/butyric-acid-fermentation/
- Zigová, Jana, et al. “Butyric acid production by Clostridium butyricum with integrated extraction and pertraction.” Process Biochemistry 34.8 (1999): 835-843.
- The Difference Between Batch, Fed-batch and Continuous Processes – https://www.infors-ht.com/en/blog/the-difference-between-batch-fed-batch-and-continuous-processes/
- He, Guo-qing, et al. “Batch and fed-batch production of butyric acid by Clostridium butyricum ZJUCB.” Journal of Zhejiang University-SCIENCE B 6.11 (2005): 1076-1080.
- Butanoic acid – C4H8O2 – https://byjus.com/chemistry/butanoic-acid/
