Borrelia burgdorferi is the causative agent of Lyme disease, mostly occurring in North America and Europe.
- It is a member of the eubacterial phylum Spirochaetes named after their spiral or wave-like body and flagella.
- B. burgdorferi is an obligate pathogen causing infections only in humans but can exist in other mammals as carriers.
- In ticks, the bacteria is transmitted from infected rodents during their larval feeding. The infected ticks then feed on a broad range of animals transferring the bacteria to new hosts.
- B. burgdorferi has an enzootic lifecycle as the bacteria transmits between Ixodes ticks and a vertebrate host. The vertebrate host can be a variety of small mammals, but infection occurs only in humans.
- The disease in humans is Lyme disease which is a zoonotic, vector-borne disease that is transmitted via the saliva of the tick to a new human host.
- The causative agent, B. burgdorferi is a Gram-negative pathogenic spirochete with a segmented genome composed of a linear chromosome and numerous linear and circular genomic plasmids.
- Over the years, new pathogenic species of Borrelia have been discovered commonly named B. burgdorferi sensu lato, including species like B. afzelii and B. garinii.
- The genus name Borrelia refers to Amedee Borrel who first made the distinction between Borrelia and other types of the spirochete.
- The species name ‘burgdorferi’ is taken from the name Burgdorfer, in the honor of Willy Burgdorfer who initially identified the species.
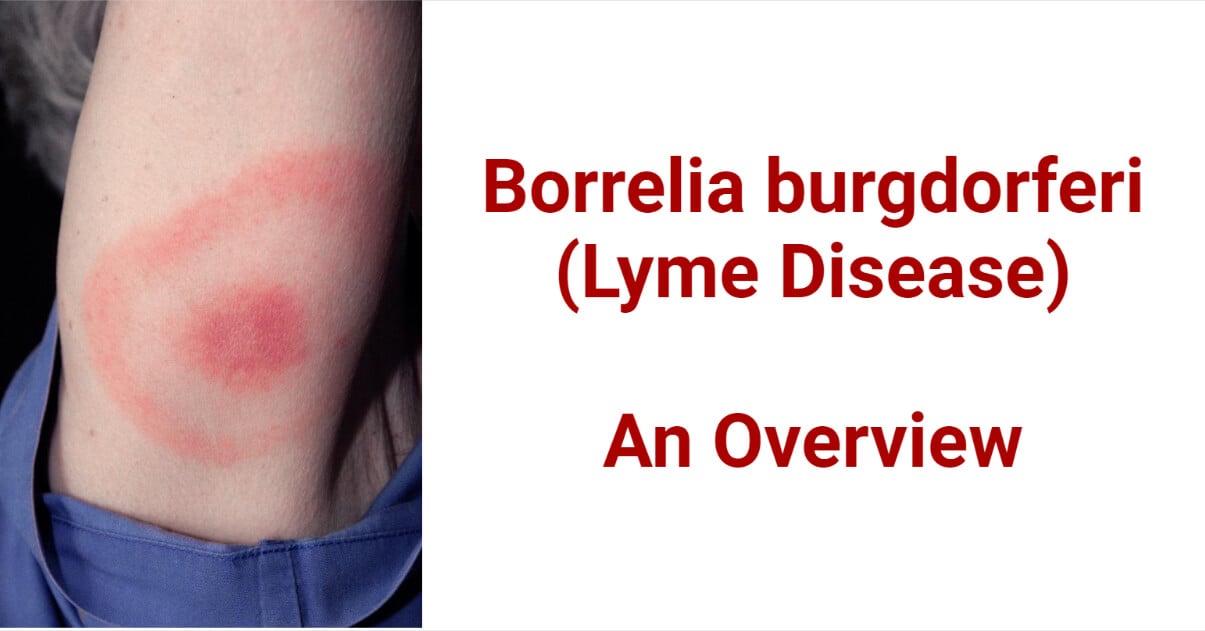
- Lyme disease is characterized by the formation of bull-eye-type red-colored rash on different parts of the body.
- Lyme disease occurs in three different stages as the infection gets severe with the progression of the disease.
- When the bacteria reach the bloodstream in great numbers, it results in relapsing fever which can persist for a long period of time.
Interesting Science Videos
Classification of Borrelia burgdorferi
- The genus Borrelia is placed in the phylum Spirochaetes that are defined by helical or spiral-shaped motile cells.
- Borrelia burgdorferi was initially defined as a new species of the genus Borrelia in 1984 based on the characterization of other species of the same genus.
- In recent years, the classification of B. burgdorferi has been modified based on its phenotypic and genotypic characteristics.
- All the species of Borrelia have been classified into two major groups; the first group containing the agents of Lyme borreliosis and the second group containing species associated with relapsing fever.
- The species belonging to the first group are designated as B. burgdorferi sensu lato consisting of various Lyme disease-causing species like B. garinii and B. afzelii. The name B. burgdorferi sensu strict is used to denote B. burgdorferi exclusively.
- The species present in the B. burgdorferi group are not equally distributed throughout the world, out of which only three are known to cause infections in humans.
- The genetic diversity within the species is due to various molecular mechanisms like mutations, deletions, a substitution that control the rate of genetic variability.
The following is the taxonomical classification of B. burgdorferi-
| Domain | Eubacteria |
| Phylum | Spirochaetes |
| Order | Spirochaetales |
| Family | Spirochaetaceae |
| Genus | Borrelia |
| Species | B. burgdorferi |
Habitat of Borrelia burgdorferi
- Borrelia burgdorferi is most prevalent in the temperate regions of the northern hemisphere in countries like North America and Europe.
- The bacteria can infect a wide range of vertebrate animals like small mammals, lizards, and birds.
- The lifecycle of B. burgdorferi is zoonotic and occurs in two different animals; insects and vertebrate hosts including humans.
- The geographic distribution of the bacteria is due to the overlapping ranges of both a competent reservoir host for B. burgdorferi and the tick vector.
- In the northern hemisphere, the primary tick species for human disease is Ixodes scapularis while that in the western states is I. pacificus.
- The role of reservoirs of B. burgdorferi in the natural environment has not been explored yet, but factors like a local and temporal variation in tick densities play a crucial role in infections.
- The circulation of B. burgdorferi in nature from the reservoir host to feeding tick larvae occurs as a part of the life cycle of the bacteria.
- The feeding larva then moults into the nymphal stage, which then feeds on a broad range of animals, including rodents.
- These animals then become new reservoirs continuing the lifecycle. The adult form of the tick feeds, particularly on larger mammals.
- The mammalian and tick host have different environments which require the bacteria to adapt to the contrasting environments.
- The ability to survive in different environments is due to the variation in gene expression, resulting in different protein components and physiological adaptation to the different environments.
- An ecological dynamic exists between the tick and B. burgdorferi influenced by host movement, availability, species composition, general vegetation characteristics, and microclimate.
Morphology of Borrelia burgdorferi
- The cells of B. burgdorferi are flexible helical shaped with dimensions ranging between 0.2-0.3 µm × 4-30 µm.
- The bacteria are mostly pleomorphic, and these can change their morphology as a response to environmental conditions.
- Peritrichous flagella are present all over the cell, providing both motility and structure to the cell. The motility of the cell is both rotational and translational, aided by the flagella.
- There are seven to eleven periplasmic flagella located at each cell end the flagella overlap at the central region of the cell.
- A multilayered outer membrane is present around the protoplasmic cylinder covered by two lipid membranes.
- Underneath the outer membrane is the cytoplasmic membrane and the enclosed cytoplasmic content.
- A periplasmic space is present between the outer and inner membrane which comprises of peptidoglycan layer and flagella filaments.
- The cell envelop of B. burgdorferi is unique and differs from that of the typical Gram-negative bacteria. Typical Gram-negative cell wall is composed of lipopolysaccharides, which are absent in the B. burgdorferi cell wall but contain immunoreactive glycolipids instead.
- Another important cellular component of B. burgdorferi is the outer surface proteins that play an important role in the transmission of the bacteria.
- The bacteria lack genes encoding enzymes required for the biosynthesis of various amino acids, fatty acids, and nucleotides.
- The genome of B. burgdorferi is composed of a small linear chromosome of 1000 kb and the G+C content of 28.6%with linear and circular plasmids that are variable in number and size.
Cultural characteristics of Borrelia burgdorferi
- Borrelia burgdorferi is a microaerophilic, fastidious bacteria that grows better on liquid media than on solid agar media.
- It requires complex nutritional requirements due to the lack of or limited biosynthetic potential of the bacteria.
- The culture medium used for the cultivation of B. burgdorferi usually contains compounds like a serum, glucose, albumin, peptides, amino acids, and vitamins.
- Besides, other nutritional requirements include N-acetylglucosamine, and long-chain saturated and unsaturated fatty acids are also required.
- The growth of B. burgdorferi occurs slowly with cells dividing every 8-12 hours during the exponential phase of growth.
- The cell density of the bacteria usually reaches about 107-108 per ml after cultivation for 5-7 days.
- Discrete colonies of B. burgdorferi can be obtained on semi-solid agar medium like Barbour-Stornner-Kelly II (BSK II) or MKP medium. The media are made selective by the addition of antibiotics like kanamycin, rifampicin, and nalidixic as selective agents.
- The colonies are subsurface colonies that do not have distinct colony morphologies; thus, it is difficult to identify the bacteria based on the growth condition and colony characteristics.
- B. burgdorferi is a lactic acid bacteria that utilize carbohydrates, especially glucose, as the major source of energy to produce lactic acid.
- The optimum temperature for the growth of the bacteria and fermentation processes is between 30-34°C with maximum growth at 33°C.
- The generation time of the bacteria is influenced by factors like culture conditions and available nutrients. The time ranges between 7-20 hours.
- The following are some cultural characteristics of B. burgdorferi on different culture media:
Borrelia burgdorferi on BSK medium
- The colonies of B. burgdorferi on the medium appear after two weeks of incubation, but three to four weeks might be required to obtain accurate enumeration and evaluation of the colony morphology.
- In general, the colonies appear like a small, white disk, but when examined with a microscope, differences in the morphology might be observed.
- The colonies are small, compact, and round with an average diameter of 0.4-0.5 mm that are mostly restricted to the surface.
- The larger diffuse colonies might also be observed with an average diameter of 0.5-0.7 mm.
- The surface of the colonies is composed of tangles of coiled spirochetes at the periphery with numerous spherical cells.
- The edges of the smaller colonies are sharp and well defined, but no isolated free spirochetes are present on the surrounding agar surface.
- The diffuse colonies, however, have fewer spherical bodies and are less tightly packed.
Biochemical Characteristics
The biochemical characteristics of B. burgdorferi are have not been studied yet due to the difficulty in growth and fastidious nature of the bacteria.
Pathogenesis of Lyme disease caused by Borrelia burgdorferi
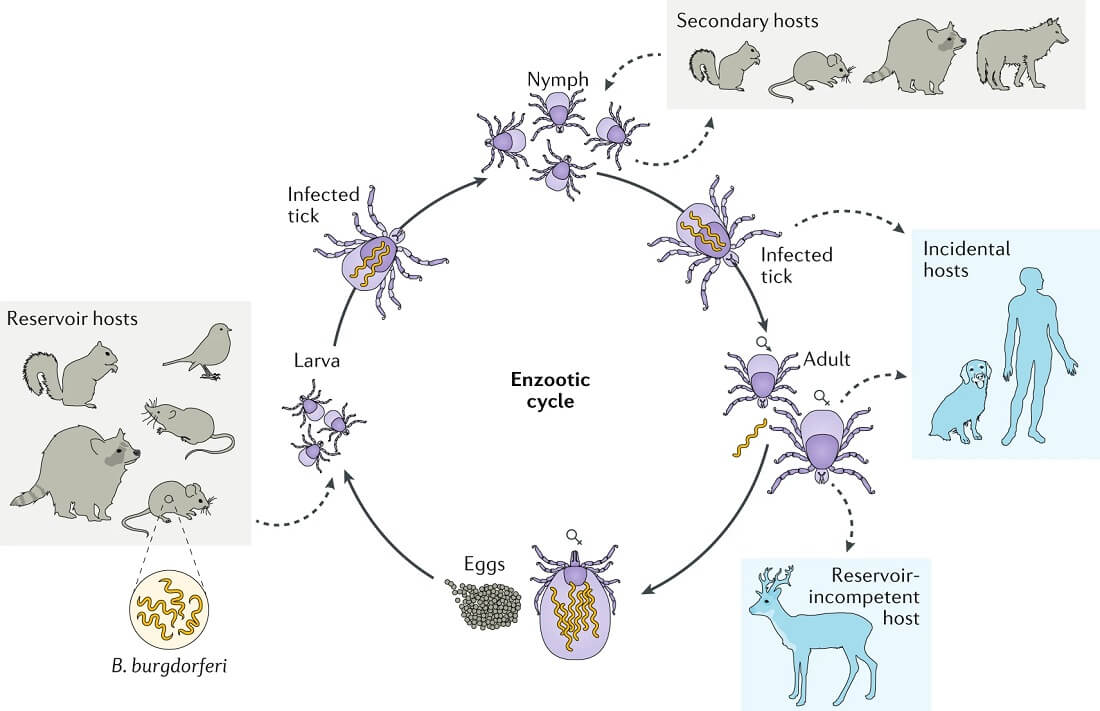
Figure: Life cycles of Ixodes scapularis and Borrelia burgdorferi. Image Source: Cheyne Kurokawa et al. 2020.
- Lyme disease is caused by the spirochete B. burgdorferi, which is transmitted by the bite of a small tick of the genus Ixodes.
- Humans are “accidental” hosts for B. burgdorferi because spirochetes from infected people are not transmitted to other hosts.
- While in the midgut of the Ixodes tick between feeding periods, the spirochetes of B. burgdorferi are in a dormant, non-replicating state attached to epithelial cells.
- There they express a plasmid-encoded, major outer surface protein (OspA), which plays a critical role in the protection of B. burgdorferi from antibodies present in the blood meal from immune hosts and may also promote tick midgut colonization by binding to tick midgut cell receptors.
- Outer surface protein A (OspA) is expressed on the surface of B. burgdorferi residing in the midgut of unfed ticks and binds specifically to gut proteins.
- Upon feeding, expression of this protein is repressed, allowing the spirochete to migrate to the salivary glands, and outer surface protein C (OspC) expression, which appears critical for transmission from ticks to mammals, is up-regulated.
- In humans, the bite of the infected tick is required for the introduction of the pathogen through healthy skin.
- This extracellular pathogen starts in the dermal tissue where it begins to adapt to life in the mammalian host by changing the expression of its surface glycoproteins.
- The change in the gene expression profile of the spirochetes in response to tick feeding, typified by the switch from OspA to OspC, is essential.
- The ability of B. burgdorferi to multiply and establish infection in the skin of the mammalian host is reflected in one of the characteristic signs of localized infection or stage 1 Lyme disease in humans, the erythema migrans (EM) rash which reflects infiltration of lymphocytes and macrophages.
- The spirochetes are highly motile and probably coated with the host protease plasmin.
- After a certain period, they are able to spread through the skin, resulting in expansion of the rash, leaving a blanched central area, and a bulls-eye appearance.
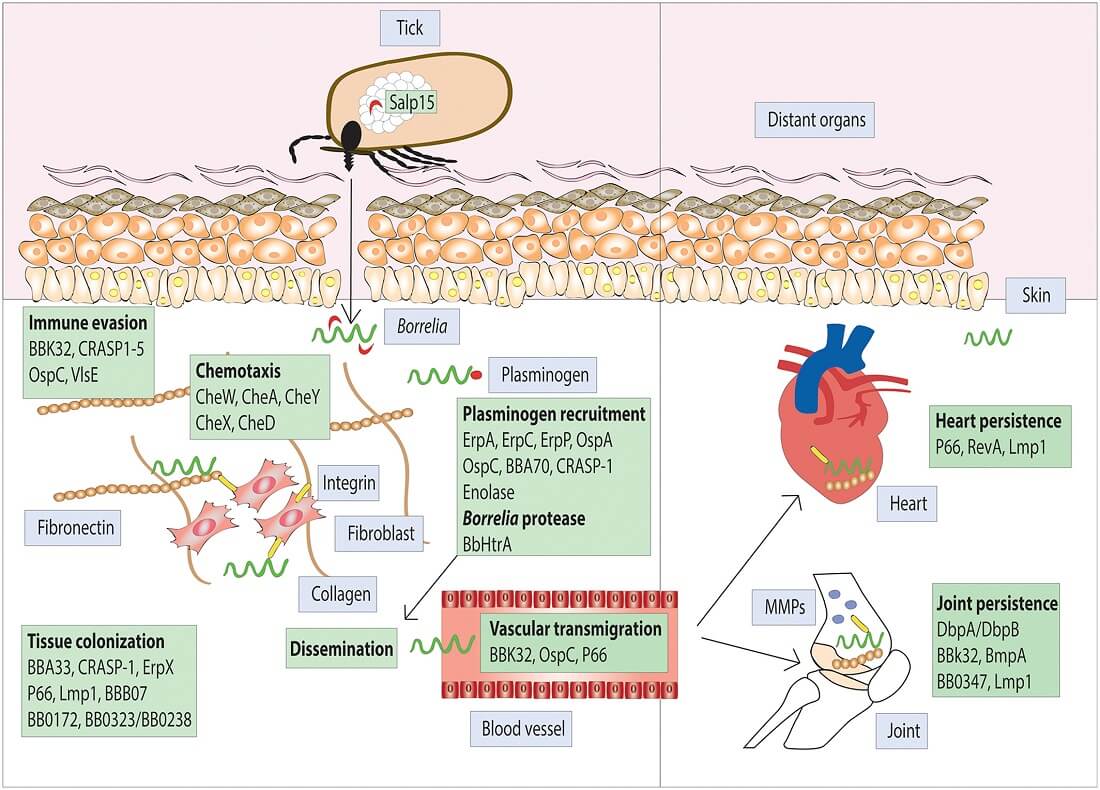
Figure: Protein interaction involved in Borrelia burgdorferi infection of mammalian hosts transmitted via Ixodes ticks. Image Source: Quentin Bernard at al. 2018.
- B. burgdorferi expresses outer surface proteins that selectively interact with endothelial cells, platelets, chondrocytes, and extracellular matrix via specific interactions with integrins, glycosaminoglycans, fibronectin, and collagen.
- These interactions are important in homing to and colonization of tissues, including the skin, joints, and heart and the bacterium also activates proteases and other induced host cell molecules to allow for dissemination through the blood and into other tissues.
- Disseminated infection (stage 2) includes transient colonization of the bloodstream.
- At this stage some degree of vascular damage, including mild vasculitis or hypercellular vascular occlusion, may be seen in multiple sites, suggesting that the spirochetes colonize the vessel wall. This stage is known as early disseminated infection or stage 2 Lyme disease.
- Later, in late-stage or stage 3 Lyme disease, the rate of bacterial multiplication appears to be significantly reduced or is kept in check by the host defenses, resulting in the very low number of bacteria present in tissues.
- The host response to B. burgdorferi plays a key role in disease pathogenesis.
- B. burgdorferi does not produce toxins or proteases that are directly responsible for tissue damage upon colonization.
- In contrast, the bacterium produces multiple molecules that activate host responses and can lead to localized and generalized inflammatory pathogenic responses.
- Most of these host responses normally function to contain or clear infections and are components of the innate defense and/or inflammatory response.
- These bacteria, however, are not eradicated by the host immune response.
- Once the human infection is established, the spirochete can survive for years, despite the development of a vigorous host immune response.
- B. burgdorferi is able to bind mammalian complement regulatory factors, which may provide resistance to complement-mediated lysis and opsonization during persistent infection of host tissues.
Clinical Manifestations of Borrelia burgdorferi
Lyme disease
- Lyme disease is characterized by three stages.
- The first stage, erythema migrans (EM), is the characteristic red, ring-shaped skin lesion with a central clearing that first appears at the site of the tick bite but may develop at distant sites as well.
- The clinical manifestations include headache, fever, muscle and joint pain, and malaise during this stage.
- The second stage, beginning weeks to months after infection, may include arthritis, but the most important features are neurologic disorders which include meningitis, neurologic deficits, and carditis.
- This is a result of the hematogenous spread of spirochetes to organs and tissues.
- In addition, neurologic symptoms and infection may occur in the meninges, spinal cord, peripheral nerves, and brain.
- The third stage is usually characterized by chronic arthritis or acrodermatitis chronica atrophicans (ACA), a diffuse skin rash, and may continue for years.
Laboratory diagnosis of Borrelia burgdorferi
Specimen
- Blood, cerebrospinal fluid, joint fluid, tissue biopsies
- Body fluids should be transported without any preservatives.
- Tissue biopsy specimens should be placed in sterile saline to prevent drying.
Direct detection methods
- The organisms can be seen directly in wet preparations of peripheral blood (mixed with equal parts of sterile, non-bacteriostatic saline) under dark- or brightfield illumination, in which the spirochetes move rapidly, often pushing the red blood cells around.
- In the case of Lyme disease, tissue sections stained with Warthin-Starry silver stain are visualized.
- PCR has detected B. burgdorferi DNA in clinical specimens from patients with early and late clinical manifestations.
- Optimal specimens include urine, synovial tissue, synovial fluid, and skin biopsies from patients with EM.
Culture
- Culture is generally not performed because it takes 6–8 weeks to complete and lacks sensitivity.
- The culture of B. burgdorferi from specimens in Barbour–Stonner–Kelly medium permits a definitive diagnosis.
- Positive cultures have been obtained only early in the illness, primarily from biopsy samples of EM lesions.
Serology
- Serology has been the mainstay for the diagnosis of Lyme disease, but 3–5% of normal people and persons with other diseases (example; rheumatoid arthritis, many infectious diseases) may be seropositive by initial EIA or indirect fluorescent antibody (IFA) assay.
- An indirect immunofluorescence test is available, but enzyme-linked immunosorbent assay (ELISA) is now widely used.
- Immunoblotting with a panel of carefully selected recombinant antigens is used to confirm serological results.
- Interpretation of the immunoblot is based on the number and molecular size of antibody reactions with the B. burgdorferi
- Blots can be analyzed for IgG or IgM.
- The antigen-antibody band patterns on the immunoblots should be interpreted with knowledge of known results from patients at various stages of Lyme borreliosis.
Treatment of Lyme disease caused by Borrelia burgdorferi
- Doxycycline is the most recommended treatment for the early stages of the disease.
- If arthritic symptoms have already appeared, longer courses of antibiotics (ceftriaxone) are used.
- Amoxicillin should be used in children and pregnant women.
- Doxycycline, amoxicillin, or cefuroxime, and parenteral cephalosporins are drugs of choice during the first stage of Lyme disease.
- Broad-spectrum cephalosporins, particularly ceftriaxone or cefotaxime, have been used successfully with patients who either fail initial treatment or are present in later stages of the disease.
Prevention and control of Lyme disease caused by Borrelia burgdorferi
- A recombinant outer surface protein A vaccine has been licensed for use in humans against Lyme disease caused by infection with organisms belonging to the B. burgdorferi complex.
- Avoiding tick-infested areas; wearing protective clothing; checking clothing, body, and pets for ticks; and removing them promptly will also assist in the prevention of infection.
- Prevention is based on the avoidance of exposure to ticks.
- Wearing long sleeves and long pants tucked into socks is recommended.
- Careful examination of the skin for ticks after being outdoors can locate ticks for removal before they transmit B. burgdorferi.
- Environmental control of ticks using the application of insecticides has provided modest success in reducing the number of nymphal ticks for a season.
- Prevention of infection also includes the use of insect repellents and wearing clothing that sufficiently protects the body from tick bites.
References
- Kurokawa, C., Lynn, G.E., Pedra, J.H.F. et al. Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol 18, 587–600 (2020). https://doi.org/10.1038/s41579-020-0400-5.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014;35(12):500-509. doi:10.1542/pir.35-12-500.
- Bernard Q, Thakur M, Smith AA, Kitsou C, Yang X, Pal U. Borrelia burgdorferi protein interactions critical for microbial persistence in mammals. Cell Microbiol. 2019 Feb;21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8. PMID: 29934966.
- Winslow C, Coburn J. Recent discoveries and advancements in research on the Lyme disease spirochete Borrelia burgdorferi. F1000Res. 2019;8:F1000 Faculty Rev-763. Published 2019 May 31. doi:10.12688/f1000research.18379.1
- Tatum R, Pearson-Shaver AL. Borrelia Burgdorferi. [Updated 2021 Jul 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532894/.
- Skar GL, Simonsen KA. Lyme Disease. [Updated 2021 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431066/.
- Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis [published correction appears in Nat Rev Dis Primers. 2017 Aug 03;3:17062]. Nat Rev Dis Primers. 2016;2:16090. Published 2016 Dec 15. doi:10.1038/nrdp.2016.90.
- Tilly K, Rosa PA, Stewart PE. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22(2):217-v. doi:10.1016/j.idc.2007.12.013.
- Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113(8):1093-1101. doi:10.1172/JCI21681.
- Corona A, Schwartz I. Borrelia burgdorferi: Carbon Metabolism and the Tick-Mammal Enzootic Cycle. Microbiol Spectr. 2015;3(3):10.1128/microbiolspec.MBP-0011-2014. doi:10.1128/microbiolspec.MBP-0011-2014.
- Dattwyler RJ, Luft BJ. Overview of the clinical manifestations of Borrelia burgdorferi infection. Can J Infect Dis. 1991;2(2):61-63. doi:10.1155/1991/902928.
- Heymann WR, Ellis DL. Borrelia burgdorferi Infections in the United States. J Clin Aesthet Dermatol. 2012;5(8):18-28.
- Sapi E, Kasliwala RS, Ismail H, et al. The Long-Term Persistence of Borrelia burgdorferi Antigens and DNA in the Tissues of a Patient with Lyme Disease. Antibiotics (Basel). 2019;8(4):183. Published 2019 Oct 11. doi:10.3390/antibiotics8040183.
- Murray TS, Shapiro ED. Lyme disease. Clin Lab Med. 2010;30(1):311-328. doi:10.1016/j.cll.2010.01.003.
- Shor S, Green C, Szantyr B, et al. Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group. Antibiotics (Basel). 2019;8(4):269. Published 2019 Dec 16. doi:10.3390/antibiotics8040269.
