- Esculin hydrolysis was first described by Rochaix in 1924.
- Swan first introduced the use of Bile Esculin Agar in 1954. Bile Esculin Agar (BEA) is a selective differential agar used to isolate and identify members of the genus Enterococcus, formerly part of the “group D streptococci”.
- The ability to hydrolyze esculin in the presence of bile is a characteristic of enterococci and group D streptococci.
- In 1970, Facklam and Moody determined that the use of the bile esculin test was a reliable way of identifying different groups of bacteria.
Interesting Science Videos
Composition of Bile Esculin Agar
| Ingredients | Gms/liter |
| Peptic digest of animal tissue | 5.00 |
| Beef extract | 3.00 |
| Esculin | 1.00 |
| Bile salts | 40.0 |
| Ferric citrate | 0.50 |
| Agar | 15.00 |
Final pH (at 25°C): 6.6±0.2
Principle of Bile Esculin Agar
This medium contains esculin, ferric citrate to provide ferric ions, and 4% oxbile to inhibit most other strains of non-group D streptococci. Peptic digest of animal tissue and beef extract serves as the source of carbon, nitrogen, and essential growth factors. Bile salts inhibit the accompanying gram-positive bacteria. Bile salts are the selective ingredient, while esculin is the differential component.
Esculin is hydrolyzed by group D streptococci and other esculin positive organisms to form dextrose and esculetin. This compound reacts with the ferric ions contained within the medium, turning the medium from its original amber color to a dark brown to black.
Thus the tolerance to the presence of bile and the hydrolysis of esculin provide the means to presumptively identify specific organisms including group D streptococci.
Preparation of Use of Bile Esculin Agar
- Suspend 64.5 grams in 1000 ml distilled water.
- Heat to boiling to dissolve the medium completely.
- Dispense into tubes or flasks.
- Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
- Allow the tubed medium to solidify in a slanted position with a butt of 2.5cm deep or pour into sterile Petri plates.
- Allow the BEA medium warm to room temperature before use.
- Inoculate and streak the medium with one isolated pure colony.
- Incubate in an aerobic atmosphere at 35ºC for 24-48 hours.
- Observe for growth and blackening of the medium.
Result Interpretation on Bile Esculin Agar
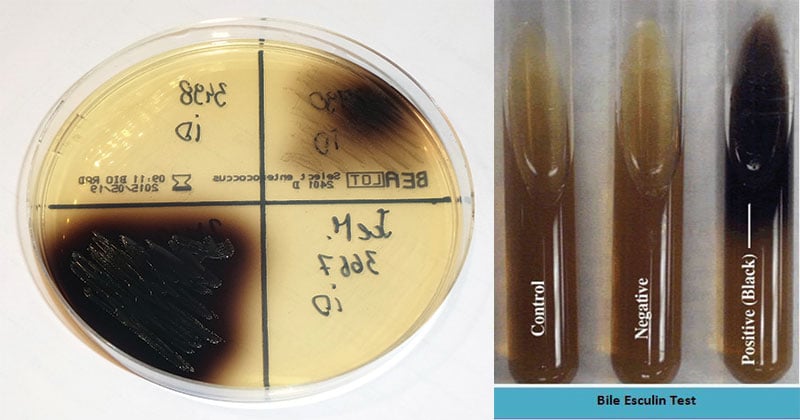
Esculin hydrolysis by an organism is indicated by a blackening of the media around the colonies.
| Organisms | Growth |
| Enterococcus faecalis | Good-luxuriant growth; Esculin hydrolysis positive resulting in blackening of the medium around growth. |
| Escherichia coli | Good growth; No blackening of media around colonies |
| Enterococcus faecium | Good-luxuriant growth; Esculin hydrolysis positive resulting in blackening of the medium around growth. |
| Yersinia enterocolitica | Good-luxuriant growth; Esculin hydrolysis positive resulting in blackening of the medium around growth. |
| Streptococcus pyogenes | None to poor growth; No blackening of media around colonies |
Uses of Bile Esculin Agar
- It is recommended for use as a differential medium in the isolation and presumptive identification of enterococci/group D streptococci.
- It is used in conjunction with other biochemical tests to identify cultures of isolated organism.
- BEA is used for the presumptive identification of Enterobacter, Klebsiella spp., and Serratia spp., among the Enterobacteriaceae.
- The use of the bile esculin test on this medium is a reliable way of identifying group D streptococci from non-group D streptococci.
- Bile Esculin Agar is recommended for the isolation and identification of Yersinia enterocolitica from food and animal feeding stuffs.
Limitations of Bile Esculin Agar
- The media is not intended for primary isolation of patient specimens. It should be used only with cultures of isolated organism.
- Bile Esculin Agar is not intended for use in the diagnosis of disease or other conditions in humans.
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- Some strains of Staphylococcus, Aerococcus and Listeria monocytogenes may grow in the presence of bile and hydrolyze esculin. L. monocytogenes will form minute black colonies.
- A heavy inoculum on BEA may cause interpretation of the bile esculin test difficult to read. Excess inoculum decreases the ability of the bile to inhibit the growth of other gram-positive organisms that may hydrolyze esculin.
- There are a few streptococci that do not hydrolyze esculin but will grow in the presence of bile. Growth without blackening of this medium does not constitute a positive test.
- BEA does not contain azide; as a result, gram-negative rods will grow on this medium. Many of these organisms may hydrolyze esculin.
References
- http://himedialabs.com/TD/M972I.pdf
- Swan, A. 1954.The use of bile-esculin medium and of Maxted’s technique of Lancefield grouping in the identification of enterococci (group D streptococci). J. Clin. Pathol. 7:160.
- Facklam, R. R., and M. D. Moody. 1970. Presumptive identification of group D streptococci: the bile-esculin test. Appl. Microbiol. 20:245
- https://foodsafety.neogen.com/pdf/acumedia_pi/7249_pi.pdf
- http://www.austincc.edu/microbugz/bile_esculin_test.php
- https://microbeonline.com/bile-esculin-test-enterococcus-species-principle-procedure-results/
- http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0888&c=UK&lang=EN

i want to ask that , i have made this media and its not solidifying, whats the reason? though i have added agar in it.