Introduction
Beverages can be defined as any drinkable liquid other than water. Commercially, there are mainly four types of beverage (hot drinks, milk drinks, soft drinks, and alcoholic drinks). However, beverages are classified into two groups: alcoholic and non-alcoholic drinks. Most of the beverages contain water, nutritive sweeteners, flavoring, coloring agents, acidification agents, emulsifying agents, etc. which influences the population of microbial growth in beverages. In beverage industries, it is always a challenge to meet the needs of consumers, the demand for safe and healthier products. Therefore, it is important to maintain all the required qualities to give consumer satisfaction.
Classification of Beverages
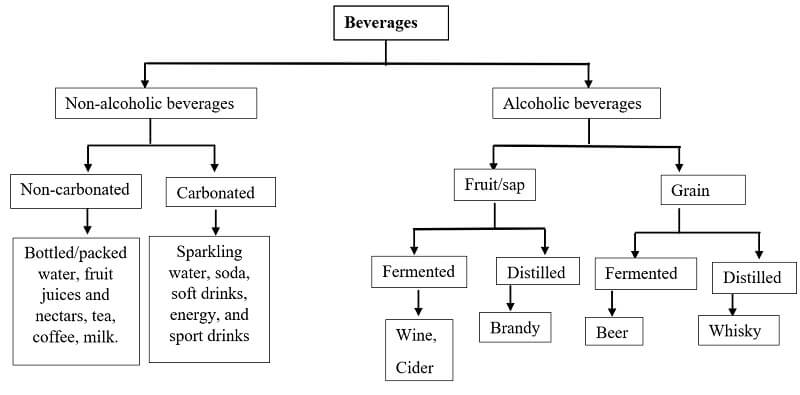
Figure- Classification of Beverages.
Interesting Science Videos
A. Non-alcoholic beverages
The non-alcoholic beverages containing water, sweeteners, acids, flavoring, coloring and emulsifying agents, and preservatives. In general, they can be classified in several ways.
- Refreshing: plain water, carbonated beverages, iced tea, and buttermilk with salt and lime juice
- Nourishing: milk – pasteurized, skimmed, evaporated, dried, malted, buttermilk, milkshakes, coffee, lemonade, etc.
- Stimulating: coffee, tea, or chocolate beverage
- Soothing: warm milk or hot tea
- Appetizing: soups, fruit juice, etc.
It can be divided into carbonated and non-carbonated beverages. Non-carbonated beverages don’t contain carbon dioxide and sparkling taste. They include fruit punch, fruit drinks, ice tea, coffee, and sports drinks. Carbonated beverages contain added carbon dioxide that gives an effervescent taste to the beverages. They include coke, soda, soft drinks, etc.
Contamination source of non-alcoholic beverages
- During the production process
- Factory environment
- Raw materials (fruits, vegetables, sugars)
- Flavorings, water, and other chemicals used
- Process machinery and filling lines in industries
- Microbiological state of the equipment
- Poor hygienic handling
- Packaging materials such as cans and bottles
- Storage conditions
Spoilage of non-alcoholic beverages
- Beverages have high water activity and often rich in vitamins and minerals so they are highly susceptible to microbial spoilage.
- The microorganisms that can resist low pH and high carbonation can grow on non-alcoholic beverages.
- Yeasts are considered to be the primary spoilage microbes. Various spoilage-causing yeasts that are found in non-alcoholic beverages include Zygosaccharomyces bailii, Saccharomyces, Brettanomyces, Hanseniaspora, Hansenula, and Pichia. Candida davenportii, C. parapsilosis, or Debaryomyces spp. Rhodotorula, Sporidiobolus, Dekkera bruxellensis, and Sporobolomyces and the black genus Aureobasidium.
- Molds grow as white, delicate, fluffy, cottony masses suspended in soſt drinks. Mold spores cannot grow in carbonated beverages but can survive. The various spoilage-causing mold that is found in non-alcoholic beverages includes Aspergillus ochraceus, Aspergillus tamarii, Aspergillus flavus, Byssochlamys nivea, Byssochlamys fulva, Paecilomyces variotii, Neosartorya fischeri, Eupenicillium brefeldianum, Phialophora mustea, Talaromyces flavus, Talaromyces trachyspermus, and Thermoascus aurantiacum. Others include Penicillium notatum, Penicillium roquefortii , Rhizopus, Cladosporium, and Fusarium, and Cladosporium spp
- Certain lactic acid bacteria (LAB) can grow in non-alcoholic beverages containing fruit juices includes Lactobacillus paracasei, L. Brevis, L. buchneri, L. Plantarum, L. perolens, and also Leuconostoc mesenteroides and Weissella
- Other various bacteria found in non-alcoholic beverages are Acetobacter, Alicyclobacillus, Bacillus, Clostridium, Gluconobacter, Lactobacillus, Leuconostoc, Saccharobacter, Zymobacter and Zymomonas.
- Pathogenic organisms Listeria monocytogenes and Y. enterocolitica E. coli and Salmonella.
The kind of defects caused by microorganisms on non-alcoholic beverages are
| Group | Genera/Species | Visual defects | Odors |
| Yeasts | Aureobasidium pullulans |
Haze, clouds, surface films, swollen packages, tainting, particulates, surface films |
Yeasty, aldehyde off-flavor, vinegar, sweet pineapple note, sweet butter, petroleum-like odor |
| Candida davenportii, C. parapsilosis, C.tropicalis, C. solani | |||
| Cryptococcus albidus, C. laurentii | |||
| Debaryomyces hansenii, D. etchellsii, D. polymorphus | |||
| Kluyveromyces lactis, K.marxianus; | |||
| Pichia anomala, P. jadinii, P.membranifaciens, P. subpelliculosa; | |||
| Rhodotorula glutinis | |||
| Saccharomyces cerevisiae, S.bayanus, S.exiguous | |||
| Zygosaccharomyces bailii, Z. bisporus, Z. lentus, Z. rouxii | |||
| Mold | Aspergillus niger, A. penicillioides, A. versicolor; Byssochlamys |
Mycelial mats, discoloration swollen packages |
Musty, stale |
| Cladosporium sphaerospermum | |||
| Fusarium oxysporum | |||
| Mucor circinelloides, M.racemosus; | |||
| Penicillium glabrum | |||
| Rhizopus stolonifer | |||
| Bacteria | LAB – Lactobacillus acidophilus, L. brevis, L. buchneri, L. paracasei, L. perolens, L. plantarum; Leuconostoc mesenteroides; Weissella confusa | Loss of carbon dioxide, ropiness, turbidity | Cheesy notes, sour, green apple |
| AAB – Acetobacter suboxydans; Gluconobacter oxydans; Gluconacetobacter sacchari; Asaia lannensis, A. bogorensis | Haze, ropiness, surface films, swollen packages | Sour, vinegar,
Antiseptic off-flavor |
|
| ACB – Alicyclobacillus acidoterrestris, A. acidophilus, A. acidocaldarius, A. cycloheptanicus, A. hesperidium, A. herbarius, A. pomorum | Difficult to detect | Antiseptic and smoky taints |
B. Alcoholic beverages
- Alcoholic beverages include a variety of products from ethnic fermented beverages, alcoholic drinks, and distilled alcohol that are available worldwide, including beer, fruit wine, refined traditional rice wine, etc.
- Alcoholic beverages are produced by the fermentation of sugars or starches in fruits or grains by naturally occurring microorganisms, or by the addition of starter cultures that biochemically change the raw materials into alcohol.
- Alcoholic beverages are generally recognized as microbiologically safe due to their high ethanol content (>4%) and low pH (<4.5).
- However, distilled products are highly stable than non-distilled products.
Contamination source of alcoholic beverages
- the raw materials containing sugars and starches
- the manufacturing processes
- the fermentation temperature (generally 18 to 35°C for 2 to 14 days) is favorable for bacterial growth (both for the starter cultures and for spoilage and pathogenic bacteria)
- Flavorings, water, and other chemicals used
- Equipment (crushers, presses, tanks, pipes, pumps, filtration units, etc)
- microbiological state of the equipment
- poor hygienic handling
- Packaging materials such as cans and bottles
- Storage conditions
Spoilage of alcoholic beverages
1. Wine
- Wine is a fermented beverage made by the alcoholic fermentation of grape or grape juice by the use of yeast with a subsequent aging process.
- Fruits other than grapes like apples, plums, peaches, pears, berries, strawberries, cherries, apricots, bananas, pineapples, cashew nuts, pomegranates, lemons, tangerines, oranges, dates, figs, etc., are also utilized for the production of wines.
- In wines, the main spoilage microorganisms are yeasts of the genera Dekkera/ Brettanomyces, Candida, Hansenula, Hanseniaspora/Kloeckera, Pichia, Saccharomyces, and Zygosaccharomyces.
- The lactic acid bacteria (LAB) Lactobacillus, Leuconostoc, and Pediococcus; and the acetic acid bacterial genera Acetobacter and Gluconobacter.
- The mold which is present in raw materials is usually eliminated during the fermentation process due to alcohol production.
The kind of defects caused by microorganisms on wine are
| Group | Genera/species | Defects |
| Yeast | Pichia, Hanseniaspora, Hansenula, Metschnikowia, Dekkera, Candida | Ester and aldehyde taints, increased volatile acidity |
| Candida, Pichia | Formation of surface slime | |
| Brettanomyces intermedius | Mousy, horsy taint, produces high levels of acetic acid | |
| Saccharomyces, Zygosaccharomyces bailii | Refermentation in the bottle | |
| Saccharomyces ludwigii, Pichia membranafaciens | Oxidized taint from acetaldehyde, flocculent masses settle as chunks and form a sliminess | |
| Schizosaccharomyces | Deactivation of wine | |
| Lactic acid bacteria | Lactobacillus brevis | Produces ethyl carbamate precursors, acidification of wine by the production of lactic and acetic acids, mannitol formation, mousy taints |
| Lactobacillus cellobiosus, Lactobacillus hilgardii | Mousy taints, bitterness due to glycerol metabolism | |
| Lactobacillus kunkeei | Production of excess acetic acid | |
| Lactobacillus trichodes | Flocculent growth | |
| Leuconostoc mesenteroides | Ropiness formation, bitterness due to glycerol metabolism | |
| Oenococcus oeni | Produces histamine, buttery flavor due to increase in diacetyl level | |
| Pediococcus parvulus | Acrolein formation from glycerol causes bitterness | |
| Pediococcus pentosaceus | Increase in viscosity due to production of polysaccharides | |
| Acetic acid bacteria | Acetobacter aceti Acetobacter pasteurianus, Gluconobacter oxydans | Oxidation of ethanol to acetaldehyde and acetic acid; production of ethyl acetate; production of acetoin from lactic acid; metabolism of glycerol to dihydroxyacetone; ropiness |
| Endo spore-forming bacteria | Bacillus spp
Clostridium spp |
An increase in acidity (butyric acid), forms sediment |
2. Beer
- Beer is a brewed beverage consisting of malt, hop, water, and yeast, which is drunk worldwide.
- It is hard to spoil due to the presence of ethanol (0.5–10% w/w), hop bitter, the high content of carbon dioxide, the low pH (3.8–4.7), and the presence of only traces of nutritive substances such as glucose, maltose, and maltotriose.
- The spoilage-causing yeast includes different genera such as Saccharomyces, Brettanomyces, Candida, Debaryomyces filobasidium, Hanseniaspora, Kluyveromyces, Pichia, Torulaspora, and Zygosaccharomyces
- Spoilage organisms are mainly lactic acid bacteria, mostly the genera Lactobacillus and Pediococcus, or they are obligate anaerobes of the species Pectinatus and Megasphaera.
- Mold species of Alternaria, Cladosporium, Epicoccum, and Fusarium are found in spoilage beer.
The main defects caused by various spoilage-causing microorganisms are
- Loss of colloidal stability
- Discoloration
- Off-texture
- Ropiness
- Abnormal attenuation rates
- Turbidity
- Taste defects
- Fermentation defect
- Defects in appearance
- Aroma defects
The defects caused by various microorganisms on beer are:
| Group | Species/Genera | Defects |
| Yeasts | Brettanomyces, Candida, and Debaryomyces filobasidium, Hanseniaspora, Kluyveromyces, Pichia, Torulaspora, and Zygosaccharomyces. | formation of phenolic, acidic, fatty acid, and estery off-flavors, as well as hazes and turbidity |
| Wild yeasts | Sulfur taints, bad eggs, and drains like off-flavor | |
| Mold | Alternaria, Cladosporium, Epicoccum and Fusarium | off-flavors ranged from burnt molasses to unclean, winey, and harsh |
| Aspergillus fumigatus | roughness and a stale flavor | |
| Lactobacillus | L. lindneri, L. brevis, L. buchneri, L. casei, L. coryneformis, L. curvatus, L. paracollinoides , L. plantarum, | Sourness and creaminess, acid production |
| Pediococci | P. damnosus, P. inopinatus, | produce optically inactive lactic acid from carbohydrates, produce acidity, cloudiness, and buttery aroma of diacetyl in |
| Gram-positive bacteria | Kocuria kristinae | producing a fruity atypical aroma |
| Gram-negative Bacteria | Pectinatus cerevisiiphilus, | Produce turbidity, propionic acid production, acetic acid and sulfur compound production, rotten eggs smell |
| Megashaera cerevisiae, M. paucivorans, M. sueciensis | Unpleasant smell. | |
| Zymomonas anaerobia | Turbidity and off-odors |
3. Cider
- Cider is an alcoholic beverage produced by the fermentation of apple juice.
- Brettanomyces spp. and Acetobacter xylinum, is found spoiling cider.
- S. ludwigii is an indigenous contaminant of cider making.
The main defects caused by various spoilage-causing microorganisms are:
- Mousiness
- Indole taint
- Sulfur and rotten-egg taints
- Discoloration
- Hazes and deposits
Preservation of Beverages
Different types of methods are applied to prevent the beverages from microbial spoilage and maintaining the nutritional and sensory quality of beverages. For the prevention of beverages spoilage caused by microbes, the methods used include thermal treatment, non-thermal treatments, use of chemical preservatives, natural preservatives, and combinations of techniques.
1. Pasteurization
- Pasteurization is a method of food preservation that involves the application of heat, usually below 100° at a certain time.
- Low-temperature long-time and high-temperature short-time treatments are the most commonly used techniques for
- Flash-pasteurization is applied to sensitive products when filling is aseptic and is usually used in carbonated drinks.
- In-pack pasteurization is applied to high acidic beverages with a high risk of spoilage.
- Hot filling is applied in beverages to lower the risk of spoilage and is often used in fruit juice drinks.
- Low-acid beverages require harsher preservative methods and are usually Ultra-high temperature pasteurization is applied.
Some examples of pasteurization applied in beverages based on pH are:
| Thermal processing | Temperature | Time |
| Flash pasteurization, pH <4.6 | 75-85 °C | 1-4 min |
| 90-96 °C | 30-90sec | |
| Hot-filling, pH<4.6 | 83-88 °C | 0.5-1.5 min |
| 92-95 °C | 10-15sec | |
| Tunnel pasteurization, pH <4.6 | 72-80 °C | 5-20 min |
| High-temperature short-time treatment, pH >4.6 | 105-115 °C | 0.5-4.2 min |
| Ultra high-temperature treatment, pH>4.6 | 130-150 °C | 1-9 sec |
2. High process processing
- It is a non-thermal pasteurization process in which liquid is subjected to high pressure in the region of 3300 – 600 MPa for about 10 minutes.
- It is applied in acidic juice and beverage products such as orange juice because pressure-tolerant spores are unable to survive in environments with low pH levels. For example
- For apple juices, 545 Mpa is applied for 1 min
- For orange juices, 241-550 MPa is applied for 3-5 min
- For blueberry juices, 400-600 MPa is applied for 15 min
- For alcoholic beverages, the juice extracted from fruits is treated with this treatment before fermentation to avoid unwanted microbial growth.
- However, it can also be applied after fermentation to avoid further growth of yeasts used for fermentation.
- During the HPP process, the beverages are sealed into their appropriate containers and placed in a stainless steel pressure chamber that contains water.
- During this process, the non-covalent bonds in microbes are disrupted without affecting the organoleptic properties of the beverages.
3. Hydrodynamic cavitation
- When the liquid is applied to this method, bubbles are formed in a fluid due to induced pressure fluctuation.
- Hydrodynamic cavitation is promising for inactivating S. cerevisiae in apple juice.
- LAB and Z. bailii were found to be eliminated when hydrodynamic cavitation was used with mild temperature treatments.
4. Pulsed electric field (PEF)
- The pulsed electric field is one of the non-thermal food preservation technologies in which food is subjected to short pulses (1-100 µs) of high electric fields with a duration of nano to milliseconds and intensity of 10 – 80 kV/cm to foods placed between two electrodes.
- PEF disrupts the microbial cell membrane, killing the microbes by lysis
- In studies, it has shown that pathogen inactivation in fruit juices when PEF was combined with antimicrobials such as citric acid, cinnamon, and bark oil.
- Lb. brevis and Z. bailii were more sensitive to PEF in alcoholic beverages.
5. Irradiation
The treatment involves exposure of beverages to X-rays, electron beams, and gamma-rays which preserve the beverages, without affecting their physical appearance. Three types of approved techniques of beverage irradiation include :
- Gamma-rays are emitted from radioactive forms of the elements cobalt and cesium (Cesium 137 and Cobalt 60)
- X-rays, generated must be at or below an energy level of 5 MeV.
- Electron beam (or e-beam), generated must be at or below an energy level of 10 MeV.
6. Membrane filtration
- Membrane filtration is a low-temperature process that uses a porous membrane with various pore sizes or a filtration to separate undesirable particles (based on their size, shape, or charge) from the fluid to retain the natural color, texture, and flavor of the beverage.
- Ultrafiltration (UF) and microfiltration (MF) are commonly used membrane filtration techniques.
- Thus membrane filtration techniques are generally used for clarification of fruit juices.
- In beverages, it is applied in apple juice clarification, fruit juice, wine, and beer clarification and concentration.
7. Preservatives
Preservatives are substances that are capable of inhibiting or retarding the growth of microorganisms. Such preservatives used in food can be divided into three types:
- Natural preservatives
- Bio preservatives
- Chemical preservatives
Preservatives that are used beverages are:
| Product | Preservative used | Effective against |
| Orange juice | Nisin with PEF(80 kV/cm, 44ºC) | Natural flora |
| Citral | L.monocytohenes | |
| Lysozyme | Salmonella typhimurium | |
| Lactoperoxidase | E.coli, Shigella spp | |
| Black pepper essential oil | Mesophilic bacteria and fungi | |
| Eugenol nanoemulsion | Mesophilic | |
| Watermelon juice | Clove essential oil | Mesophilic |
| Clove essential oil | ||
| Lemon juice | Lemon essential oil | A.acidoterrestris spores |
| Pineapple juice | Lemongrass essential oil | E.coli, L.monocytogenes, S. enteritidis |
| Apple juice | Cinnamon powder and essential oil | Listeria monocytogenes |
| Lactoperoxidase | E.coli,Shigella spp | |
| Chitosan | Yeast and molds | |
| Betel leaf essential oil | Mesophilic bacteria and fungi | |
| Nisin combined with carvacrol or citric acid | C.sakazakii and E.coli | |
| Clove oil, lime oil, oregano oil | E.coli | |
| Carrot juice | Combination of carvacrol and p-cymene | V.cholera |
| Tomato juice | thymol | C.lusitaniae |
| Mixed fruit juice(apple and orange) | Combination of mint and lemongrass essential oil or eucalyptus essential oil with thermal treatment(70°C or 80 °C for 30-90 sec) | |
| Fresh-made apple juice and apple ciders | Enterocin | B.licheniformis |
| Apple cider | Sulfites | Gram-negative bacteria |
| Enterocin with heat(95°C) | B.licheniformis | |
| Nisin or cinnamon with PEF(90kV/cm, 20 µs, 42ºC) | E.coli | |
| Unpasteurized apple juice | chitosan | E.coli and S.typhimurium |
| Wine | Chitosan and SO2 | Acetic acid bacteria |
| Sorbic acid | Prevent yeast fermentation | |
| Sulfites | Acetobacter spp | |
| Beer | Chitosan | Lactic acid bacteria and yeast |
| Benzoic acid | Yeast and bacterial | |
| Sorbic acid | Prevent yeast fermentation |
References
- Bartowsky, E. J. (2009). Bacterial spoilage of wine and approaches to minimize it. Letters in Applied Microbiology, 48(2), 149–156. https://doi.org/10.1111/j.1472-765X.2008.02505.x
- Cosme, F., Vilela, A., Filipe-Ribeiro, L., Inês, A., & Nunes, F. M. (2018). Wine Microbial Spoilage: Advances in Defects Remediation. In Microbial Contamination and Food Degradation. Elsevier Inc. https://doi.org/10.1016/b978-0-12-811515-2.00009-3
- Doyle, M. P. (2009). Food Microbiology and Food Safety Series Editor. Retrieved May 2, 2021, from http://www.springer.com/series/7131
- du Toit, M., & Pretorius, I. S. (2019). Microbial Spoilage and Preservation of Wine: Using Weapons from Nature’s Own Arsenal -A Review. South African Journal of Enology & Viticulture, 21(1). https://doi.org/10.21548/21-1-3559
- Esmaeili, S., Mogharrabi, M., Safi, F., Sohrabvandi, S., Mortazavian, A. M., & Bagheripoor-Fallah, N. (2015). The common spoilage microorganisms of beer: Occurrence, defects, and determination-a review. Carpathian Journal of Food Science and Technology, 7(4), 68–73.
- HAWTHORN, J. (1969). Organisms in Foods. In Nature (Vol. 224, Issue 5215). https://doi.org/10.1038/224196b0
- Hutzler, M., Riedl, R., Koob, J., & Jacob, F. (2012). Fermentation and spoilage yeasts and their relevance for the beverage industry – A review. BrewingScience, 65(3–4), 33–52.
- Jarvis, B. (2014). Cider (Cyder; Hard Cider). Encyclopedia of Food Microbiology: Second Edition, March, 437–443. https://doi.org/10.1016/B978-0-12-384730-0.00066-5
- Jay, J. M. (2000). Modern food microbiology Sixth edition.
- Jeon, S. H., Kim, N. H., Shim, M. B., Jeon, Y. W., Ahn, J. H., Lee, S. H., Hwang, I. G., & Rhee, M. S. (2015). Microbiological diversity and prevalence of spoilage and pathogenic bacteria in commercial fermented alcoholic beverages (beer, fruit wine, refined rice wine, and yakju). Journal of Food Protection, 78(4), 812–818. https://doi.org/10.4315/0362-028X.JFP-14-431
- Juvonen, R., Virkajärvi, V., Priha, O., & Laitila, A. (2011). Microbiological spoilage and safety risks in non-beer beverages. In VTT Tiedotteita – Valtion Teknillinen Tutkimuskeskus (Issue 2599).
- Kaur, P., Ghoshal, G., & Banerjee, U. C. (2019). Traditional bio-preservation in beverages: Fermented beverages. In Preservatives and Preservation Approaches in Beverages: Volume 15: The Science of Beverages (Issue August). Elsevier Inc. https://doi.org/10.1016/B978-0-12-816685-7.00003-3
- Kregiel, D. (2015). Health Safety of Soft Drinks: Contents, Containers, and Microorganisms. https://doi.org/10.1155/2015/128697
- N Silva, M. M., Pereira, K. S., & Alice Coelho, M. Z. (n.d.). Food additives used in non-alcoholic water-based beverages– a review. https://doi.org/10.15406/jnhfe.2019.09.00335
- Nychas, G.-J. E., & Panagou, E. (2011). Microbiological spoilage of foods and beverages. In Food and Beverage Stability and Shelf Life. Woodhead Publishing Limited. https://doi.org/10.1533/9780857092540.1.3
- Olin, M., Rasilainen, K., Itälä, A., Pulkkanen, V.-M., Matusewicz, M., Tanhua-Tyrkkö, M., Muurinen, A., Ahonen, L., Kataja, M., Kekäläinen, P., Niemistö, A., Laitinen, M., & Martikainen, J. (n.d.). Microbiological spoilage and safety risks in non-beer beverages produced in a brewery environment. Retrieved May 15, 2021, from http://www.vtt.fi/publications/index.jsp
- Pandey, A., & Negi, P. S. (2018). Use of Natural Preservatives for Shelf Life Extension of Fruit Juices. In Fruit Juices: Extraction, Composition, Quality, and Analysis. Elsevier Inc. https://doi.org/10.1016/B978-0-12-802230-6.00029-1
- Raczek, N. N. (2004). Food and beverage preservation. Directory of Microbicides for the Protection of Materials, 287–304. https://doi.org/10.1007/1-4020-2818-0_16
- Ray, B. (2005). Food microbiology laboratories. In Nutrition & Food Science (Vol. 35, Issue 1). https://doi.org/10.1108/nfs.2005.01735aab.015
- Sakamoto, K., & Konings, W. N. (2003). Beer spoilage bacteria and hop resistance. International Journal of Food Microbiology, 89(2–3), 105–124. https://doi.org/10.1016/S0168-1605(03)00153-3
- Sayed, A. (2018). The Beverages. Agricultural Research & Technology: Open Access Journal, 14(5), 1–9. https://doi.org/10.19080/artoaj.2018.14.555933
- Vara, S., Kumar, M., Bhavya, K., & Dwarapureddi, K. (2019). Natural preservatives for nonalcoholic beverages. Preservatives and Preservation Approaches in Beverages: Volume 15: The Science of Beverages, January, 179–201. https://doi.org/10.1016/B978-0-12-816685-7.00006-9
- Vasantha Rupasinghe, H. P., & Juan, L. (2012). Emerging Preservation Methods for Fruit Juices and Beverages. In Food Additive. InTech. https://doi.org/10.5772/32148
- Wareing, P., & Davenport, R. R. (2007). Microbiology of Soft Drinks and Fruit Juices. Chemistry and Technology of Soft Drinks and Fruit Juices: Second Edition, October, 279–299. https://doi.org/10.1002/9780470995822.ch11.

Hi Dear Sir,
I am khalid stanikzai and I need a food microbiology for lab analysis and meanwhile for food physiochemistry analysis, so please help with any website, pdf or better manuals.
by regards