Agrobacterium is a phytopathogen that infects plants through wound sites, causing crown gall disease, and is one of the most popular plant transformation tools used in agriculture to date.
- Agrobacterium tumefaciens is the soil pathogen that utilizes its bacterial type IV secretion system for the transfer of its transferred (T)-DNA into the host cells.
- The genus Agrobacterium consists of different species depending on their disease symptomology and host range. Some of the species of Agrobacterium include A. radiobacter, A. vitis, A. rhizogenes, A. rubi and A. tumefaciens.
- The organisms of this genus are most notably known as plant transformation tools used in a wide range of host cells.
- The host range of the bacteria is determined by different bacterial as well as plant factors. Bacterial factors include virulence genes and T-DNA oncogenes, whereas the plant factors include genes required for transformation and tumor formation.
- The natural diversity of the bacteria is determined based on the presence of the primary pathogenic determinant, the Ti/Ri plasmid.
- Different strains of Agrobacterium can be isolated from all around the world in a wide range of host plants. Some of the common host plants include roses, poplar, weeping fig, chrysanthemum, and other fruit trees.
- The presence of different plasmids and the ability of the organism to transfer a DNA segment with the tumor-inducing plasmid into the genome of the host cell is the primary factor behind the use of Agrobacterium in plant transformation.
- Agrobacterium is a Gram-negative rod-shaped bacterium ranging in size from 1.5 to 3 µm in length and 0.6 to 1.0 µm in width. The bacterium is motile with one or as many as six flagella.
- These do not form spores and are strictly aerobic organisms residing in soil with clinical as well as biotechnological applications.
Interesting Science Videos
Factors affecting Agrobacterium-mediated Gene Transfer
- There are a number of methods that are employed to obtain transgenic plants, some of which include Agrobacterium-mediated transformation, particle bombardment, protoplasts mediated by polyethene glycol, and liposome-mediated transformation.
- Among all these methods, Agrobacterium-mediated transformation results in single-copy transgenes, which are comparatively more stably expressed than multiple gene copies.
- The process is, however, influenced by different factors like bacterial strains and cell density, plant species, plant growth regulators, and environmental factors.
- In order to develop an efficient transformation protocol, it is necessary to find the right combination of these factors.
The following are some of the factors that influence Agrobacterium-mediated transformation;
a. Explants
- Explants are target material for Agrobacterium-mediated transformation, which can be embryonic cultures, immature embryos, mature seed-derivatives, leaf blades, and even stem segments.
- The selection of explants should be made so as to ensure the recovery of the whole transgenic plant.
- Different studies have taken place to determine the best explants for an efficient transformation process.
- Embryonic callus derived from mature seeds is considered one of the best explants for Agrobacterium-mediated transformation in certain plant species.
- It has been discovered that one of the essential factors that enhance transformation is the desiccation of explants prior to or after Agrobacterium infection.
- The differences in the efficiency of the transformation on different plant tissues have been attributed to the differences in the ability of the bacteria to attach to the plant cells and the differences in the T-DNA transfer mechanism.
b. Explants Wounding
- Wounding of explants is required for the efficient Agrobacterium-mediated transformation.
- The type and method of wounding can range from simple wounds during explants preparation to particle gun-mediated micro-wounding.
- There are other forms of wounding that include Agrobacterium-filled syringes and sonication.
- Transformation is also enhanced by the formation of micro wounding on the surface and sub-surface layers of targeted tissues, resulting in the release of phenolic compounds.
- The efficiency of transformation also depends on the application of additional phenolic substances.
c. Plant species and Genotype
- The differences in the efficiency of Agrobacterium-mediated transformation in different plant species are due to the differences in inducer molecules.
- The success of transformation depends on the chromosomal and plasmid genomes that encode all materials required for attachment and DNA transfer.
- Different plants have different levels of vir gene expression in different hosts, which affects the sensitivity to infection by Agrobacterium.
- Even within the same species, several cultivars or ecotypes exhibit varying degrees of susceptibility to tumorigenesis by Agrobacterium species.
- Most of the Agrobacterium-mediated transformations are performed on dicotyledonous species, but more recently, the frequency of gene transfer in monocotyledonous species has increased.
d. Antibiotics
- During transformation, the cocultivation is followed by the suppression of bacteria so as to not interfere with the growth and development of the host plant.
- The elimination of the bacteria is achieved by the use of one or more antibiotics in the culture medium.
- Some of the commonly used antibiotics are carbenicillin and cefotaxime. The type of antibiotics used also depends on the plant species and the Agrobacterium strains.
- It is essential to determine the correct ratio of antibiotics to achieve antibiotic selection and an adequate rate of tumorigenesis.
e. Plant Growth Regulators (PGR)
- Plant transformation also requires the addition of plant growth regulators, and the correct choice of regulators is one of the most important factors affecting the process.
- The competence and susceptibility to Agrobacterium infection in recalcitrant explants is either low or absent in the absence of PGR treatments.
- The presence of 2,4-D in the growth medium during the cocultivation process is known to enhance transformation efficiency.
- The use of growth regulators facilitates cell division and differentiation in many tissues; however, the use of regulators should be done at a particular stage of the plant cell cycle.
f. Light
- Light is an important factor that affects the efficiency of Agrobacterium-mediated transformation as light affects different physiological factors in the plant like the plant hormone levels, cell proliferation, and cell cycle stage.
- Light has also been known to increase the amount of the phenolic vir gene inducer, which affects the transformation process as it regulates the T-DNA transfer.
- Various Agrobacterium-mediated transformation processes utilize dark co-culture conditions to improve the morphology of the explants.
- Some studies have also indicated that the effect of light on the process is primarily based on the photoperiod.
g. Temperature
- Early studies related to the efficiency of Agrobacterium-mediated transformation indicate that high temperature was detrimental to tumor development.
- A temperature of about 32°C is known to suppress tumor development due to conformational changes in the virA genes. The optimal temperature for the transfer of T-DNA was found to be 19°C.
- The optimal temperature for the transfer might differ in different species, but the temperature range of 19°C to 22°C was considered ideal for many plant species.
h. Agrobacterium Strains
- The infecting ability of different Agrobacterium strains is different depending on the presence of different plasmids.
- The most efficient group of bacteria used for transformation is the combination of a standard binary vector in a super-virulent strain and a super binary vector in a regular strain.
- Different combinations of Agrobacterium strains might be used for different species of plant cells.
Principle of Agrobacterium-mediated Gene Transfer
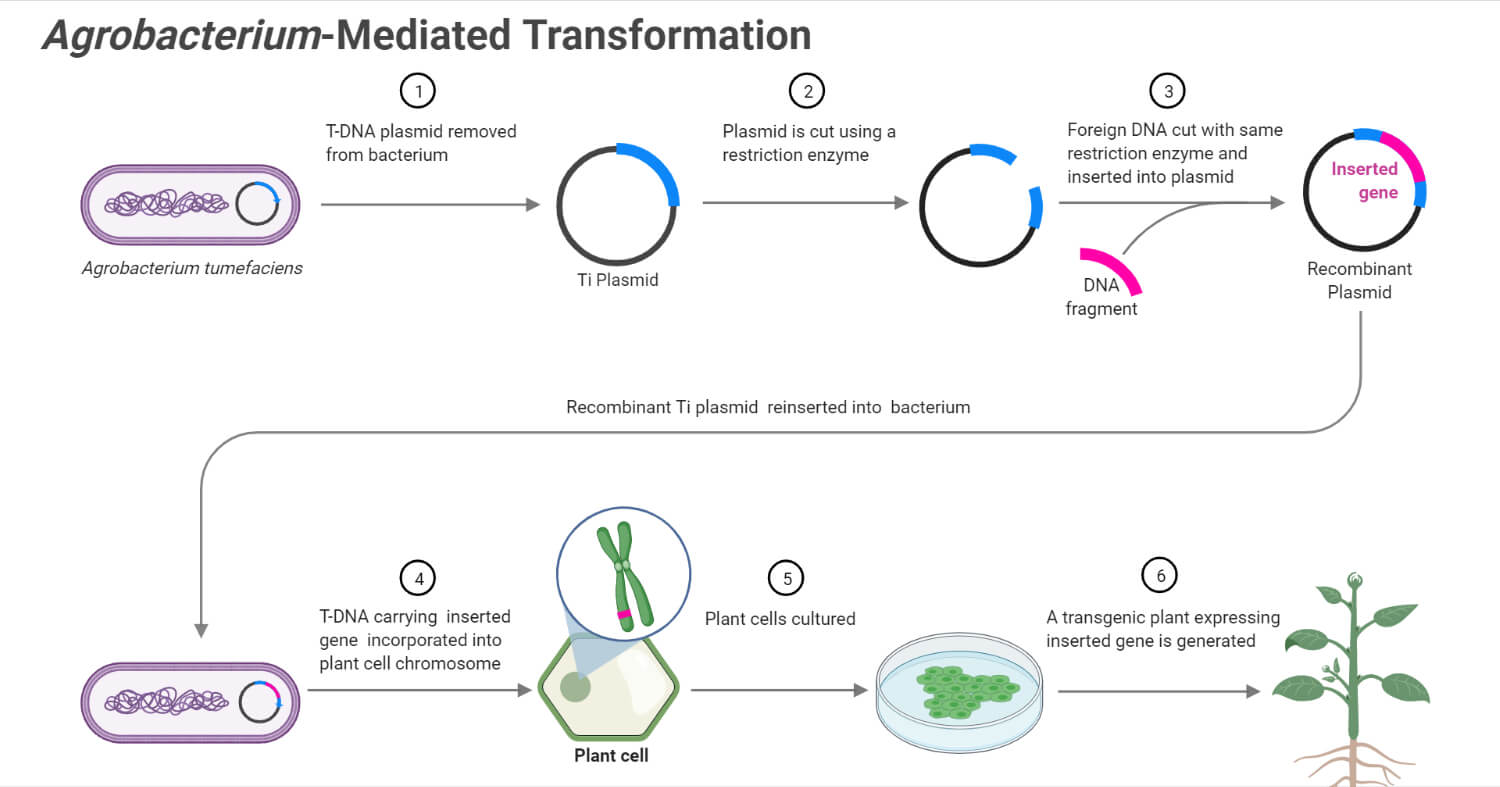
- The basis of Agrobacterium-mediated transformation is the ability of the organism to transfer its T-DNA into the host cells efficiently.
- The biology of the process consists of two components; the T-DNA consists of 25 bp repeats that end at the T-region and the virulence (vir) region composed of seven major loci.
- The mechanism of Agrobacterium-mediated transformation is based on the transfer of a piece of plasmid by the bacteria into the plant cells during infection.
- The plasmid then integrates into the nuclear genome in order to express its own genes and affect the hormonal balance in the host cell.
- Besides, the bacteria also produce a number of enzymes that are involved in the synthesis of opines that is then used by the bacteria as nutrients.
- Some of the essential components of the bacteria involved in infection are T-DNA present on the plasmid called Ti (tumor-inducing) plasmid along with other functional components like virulence (vir), conjugation (con), and origin of replication (ori).
- The infection begins with the entry of the bacteria through wounded sites. The binding of bacteria to the plant cells is enhanced by the release of phenolic acetosyringone (AS) by the injured plant cells.
- The AS activates the VirA proteins on the bacteria, which activates VirG via phosphorylation of its aspartate residue.
- The activated form of VirG then binds to other vir genes, inducing their expression. VirD activated by this process stimulates the T-strand generation (a single-stranded copy of the T-DNA).
- The VirD2 covalently binds to the 5’ end of the T-strand as the 5’ end is the leading end during the transfer. Other factors like VirE2 and VirB proteins also bind to the T-strand, forming a T-complex.
- The complex is then passed into the nucleus by the nuclear target signals released by the Vir proteins. The T-DNA strand is integrated into the plant genome randomly as either a single copy or multiple copies.
- The integration usually occurs in the transcription active or repetitive regions of the genome by the process of recombination.
- Even though much is known about the molecular biology of T-DNA transfer in Agrobacterium cells, not much is known about the plant-encoded factors involved in the process.
Requirements (Materials and Reagents)
Materials/ Equipment
- Sterile 50 ml plastic tubes
- Autoclave
- Controlled Tissue Culture Rooms at 25°C with 16/8 hr light/dark period
- Shaker Incubator
- Vacuum pump
- Laminar hood for tissue culture
- Glassware (Beakers, cylinders, Petri dishes, Duran bottles, and Flasks)
- Filter paper
- Parafilm
- Forceps and Scalpel
- Pipettes
- Centrifuge
- Spectrophotometer
- Tissue culture vessels
- Surgical blades
Reagents
- Explant (Stems, embryo, cotyledons, or other tissues)
- Agrobacterium strain
- 13% Sodium hypochlorite
- B5 Medium
- Agar
- Tryptone
- Yeast Extract
- Sodium Chloride
- 35% Hydrochloric acid
- Sterile distilled water
- 75% Ethanol
- Sucrose
- Abscisic Acid
- Rifampicin
- Kanamycin monosulfate
- Gellan gun powder
- PCR primer star Mix
- Carbenicillin disodium salt
Media Preparation for Agrobacterium-mediated Gene Transfer
a. LB medium for Agrobacterium culture
- 5 grams of yeast extract, 10 grams of tryptone, and 5 grams of sodium chloride are dissolved in 1 liter of distilled water.
- To 50 ml of the LB medium, 50 µl of 100 mg/ml rifampicin stock and 50 µl of 50 mg/ml kanamycin stock are added.
b. Murashige and Skoog medium for seed germination
- 4.43 gram of Murashige and Skoog basal medium power and 3 gm of sucrose are added to 1 liter distilled water.
- To this, 2.5 gm gellan gun powder is added and autoclaved. 25 ml of this medium is poured on Petri plates under laminar flow.
c. Cocultivation medium
- To the Murashige and Skoog basal medium, 750 µl of 2 mg/ml BAP is added. Again, 500 µl of 2mg/ml ABA is added to the medium.
- 25 ml of the medium is poured onto sterile Petri plates in the laminar flow.
d. Shooting Medium
- Shooting medium is prepared by adding 50 µl of 50 mg/ml kanamycin and 2.5 ml of 200 mg/ml carbenicillin to the cocultivation medium.
e. Rooting Medium
- Rooting medium is prepared by adding 50 µl of 50 mg/ml kanamycin and 1 ml of 200 mg/ml carbenicillin.
Procedure or Protocol of Agrobacterium-mediated Gene Transfer
The protocol or procedure for the Agrobacterium-mediated transformation might differ depending on the type of explants selected for the process. The following is the protocol for Agrobacterium-mediated transformation in the case of an embryo;
a. Sterilization and Germination of seeds
- The seeds are sterilized with Cl2 for 1-2 hours, and the seeds are then soaked in water for 2 hours at room temperature in a Petri dish.
- The seed coats are removed with forceps from the seed and are further sterilized with 75% ethanol for 30 seconds. These are then rinsed with 20 ml of the 3% sodium hypochlorite.
- The sterilized seeds are placed on Petri plates containing seed germination medium and are incubated at 28°C for 2 days in the dark. Each plate can contain about 15-25 seeds.
b. Inoculum Preparation
- 2 ml of the LB medium containing rifampicin and kanamycin is inoculated with a single Agrobacterium colony. The culture is then incubated in the shaker incubator at 28°C.
- The Agrobacterium culture prepared is centrifuged at 4000g for 10 minutes. The supernatant is removed, and the pellets are cleaned with a 1 ml MS liquid medium.
c. Preparation of the explants
- The seeds are pulled out of the germination medium and are placed on empty sterile Petri plates with a stack of filter papers.
- The radicle is removed, and the seed is cut to remove ½ of the cotyledons and endopleura.
- The cotyledons are separated with a sterile scalpel and are wiped off. The detached cotyledons are collected into the MS liquid medium in a sterile glass beaker.
- The MS liquid medium containing the cotyledons are poured into MS liquid medium containing Agrobacterium and shaken gently.
- The glass beaker is covered with a container and sealed with film, and ut into the desiccators attached to the vacuum pump.
- The explants are left on the medium for 5 minutes after two sessions of vacuum infiltration.
- A sterile filter paper is packed on the cocultivation medium, and the infected explants are placed on the filter paper with forceps. The adaxial surface of the cotyledon is to be kept upwards.
- The Petri plates are then sealed and incubated in the dark for 2 days at 28°C.
d. Shoot Initiation
- The explants are then transferred to the shooting medium containing kanamycin and carbenicillin in order to inhibit the growth of Agrobacterium.
- Every plate can contain 5-6 explants that are then incubated under light for 2-3 weeks at 25°C.
e. Regeneration
- Once the shoots begin to appear on the explants, these are pulled out and placed on sterile Petri plates with a stack of filter paper.
- The shoots are cut off with a sterile scalpel, and the embryoid part of the shoots are removed.
- The shoots are then transferred to a 100 ml glass flask or tissue culture vessels with a rooting medium.
- About 3-4 shoots can be added per vessel. The vessels are incubated under light for 1-2 weeks at 25°C.
- After 2 weeks, if no roots are observed, the unfolded leaves and end parts of the shoots are cut off, and the shoots are transferred to a new rooting medium.
f. Plant acclimation
- After the roots begin to appear, the over on the flasks are loosened and further incubated at 25°C for 3 days.
- The plants are then pulled out from the medium and washed off with running water.
- These are then transferred to pots filled with wet compost and watered.
- The pots are covered with zip bags to keep the moisture. These are incubated in a good condition chamber under light at 25°C for 1-2 weeks.
- Once the plants grow in good condition, the zip bags are removed, and the plants are watered.
Applications of Agrobacterium-mediated Gene Transfer
The following are some of the important applications of Agrobacterium-mediated transformation;
- The Agrobacterium-mediated transformation has been used as a method of genetic modification of plants for the production of various substances like proteins, antibodies, and even vaccines.
- Different plants have also been modified to produce life-saving pharmaceutical products like anticoagulants, human epidermal growth factors, and interferons.
- Transgenic plants prepared with Agrobacterium serve as biomonitors to detect the presence of toxic compounds in the environment as well as to detoxify the contaminated soil and water.
- Agrobacterium-mediated transformation has also remarkably increased crop yields by modifying the shelf-life and biosynthesis of the plants.
- Plants can be modified to enhance tolerance against biotic and abiotic factors, nutrient capture with increased pest resistance.
- Agrobacterium-mediated transformation has been used to produce insect resistance crops by the incorporation of various toxic genes like the Bt toxin genes.
- The increase in pest resistance results in a reduction in the use of harmful agrochemicals and herbicides.
- Agrobacterium-mediated transformation is one of the less complicated genetic engineering techniques which has the possibility of being upgraded to use with other organisms as well.
Limitations of Agrobacterium-mediated Gene Transfer
Even though Agrobacterium-mediated transformation has been advancing over the years with much success, there are some problems and limitations associated with this technique. Some of the commonly encountered limitations and problems with the technique are;
- The most important limitation associated with this technique is the narrow host range, as it is still limited to particular plant species.
- Even though a lot is known about the mechanism of T-DNA transfer in the bacteria, not much is known about the plant-encoded factors that affect the efficiency of this process.
- The technique is labor-intensive as it requires the development of plant regeneration protocols and detailed time-consuming processes. Many of these processes are prone to in vitro variations, resulting in unfavorable results.
- The success of transformation in the case of monocots depends on the use of embryos as the explants; however, these are only available for a short period of time during the year.
- Agrobacterium-mediated transformation cannot transfer large DNA molecules into more economically important plants, which indicates a possible introduction of a powerful vector system.
References
- Sivanandhan, G., Arunachalam, C., Vasudevan, V. et al. Factors affecting Agrobacterium-mediated transformation in Hybanthus enneaspermus (L.) F. Muell.. Plant Biotechnol Rep 10, 49–60 (2016). https://doi.org/10.1007/s11816-016-0385-8
- Otten L., Burr T., Szegedi E. (2008) Agrobacterium: A disease-causing bacterium. In: Tzfira T., Citovsky V. (eds) Agrobacterium: From Biology to Biotechnology. Springer, New York, NY. https://doi.org/10.1007/978-0-387-72290-0_1
- Gelvin, Stanton B. “Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool.” Microbiology and molecular biology reviews : MMBR vol. 67,1 (2003): 16-37, table of contents. doi:10.1128/mmbr.67.1.16-37.2003
- Hwang, Hau-Hsuan et al. “Agrobacterium-mediated plant transformation: biology and applications.” The arabidopsis book vol. 15 e0186. 20 Oct. 2017, doi:10.1199/tab.0186
- Nester, Eugene W. “Agrobacterium: nature’s genetic engineer.” Frontiers in plant science vol. 5 730. 6 Jan. 2015, doi:10.3389/fpls.2014.00730
- Li, W., Guo, G. & Zheng, G. Agrobacterium-mediated transformation: state of the art and future prospect. Chin.Sci.Bull. 45, 1537–1546 (2000). https://doi.org/10.1007/BF02886209
- Jones, H.D., Doherty, A. & Wu, H. Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods 1, 5 (2005). https://doi.org/10.1186/1746-4811-1-5
- Alicja Ziemienowicz. Agrobacterium-mediated plant transformation: Factors, applications and recent advances. Biocatalysis and Agricultural Biotechnology. Volume 3, Issue 4. 2014. Pages 95-102. https://doi.org/10.1016/j.bcab.2013.10.004.
- Risha Amilia Pratiwi and Muhammad Imam Surya (April 26th 2020). Agrobacterium-Mediated Transformation, Genetic Transformation in Crops, Kin-Ying To, IntechOpen, DOI: 10.5772/intechopen.91132. Available from: https://www.intechopen.com/books/genetic-transformation-in-crops/Agrobacterium-mediated-transformation
- Ziemienowicz, A. (2014). Agrobacterium-mediated plant transformation: Factors, applications and recent advances. Biocatalysis and Agricultural Biotechnology, 3(4), 95–102. doi:10.1016/j.bcab.2013.10.004
- zhenxian zhang, Xin Li, Si Ma et al. A Protocol for Agrobacterium-mediated Transformation of Cucumber (Cucumis sativus L.) from cotyledon explants, 19 September 2017, PROTOCOL (Version 1) available at Protocol Exchange [https://doi.org/10.1038/protex.2017.107]
- Karami O (2008). Factors affecting Agrobacterium-mediated Transformation of Plants. Transgenic Plant Journal. September, 2008.

this really helped thank you