Water has been the source of human life on Earth. Water plays a crucial role in maintaining the quality of human life. 30% of the world’s population lacks access to clean, potable, and reliable drinking water, according to the United Nations. Water purification is necessary to provide and supply clean and safe water from harmful/ contaminated water.
Sources of Water Pollution
Water pollution is of two types: Natural and Man-made (including urbanization and industrialization). The sources of water pollution are:
- Agricultural pollutants
- Sewage
- Industrial waste products
- Physical pollutants (heat, radioactive substances)
Interesting Science Videos
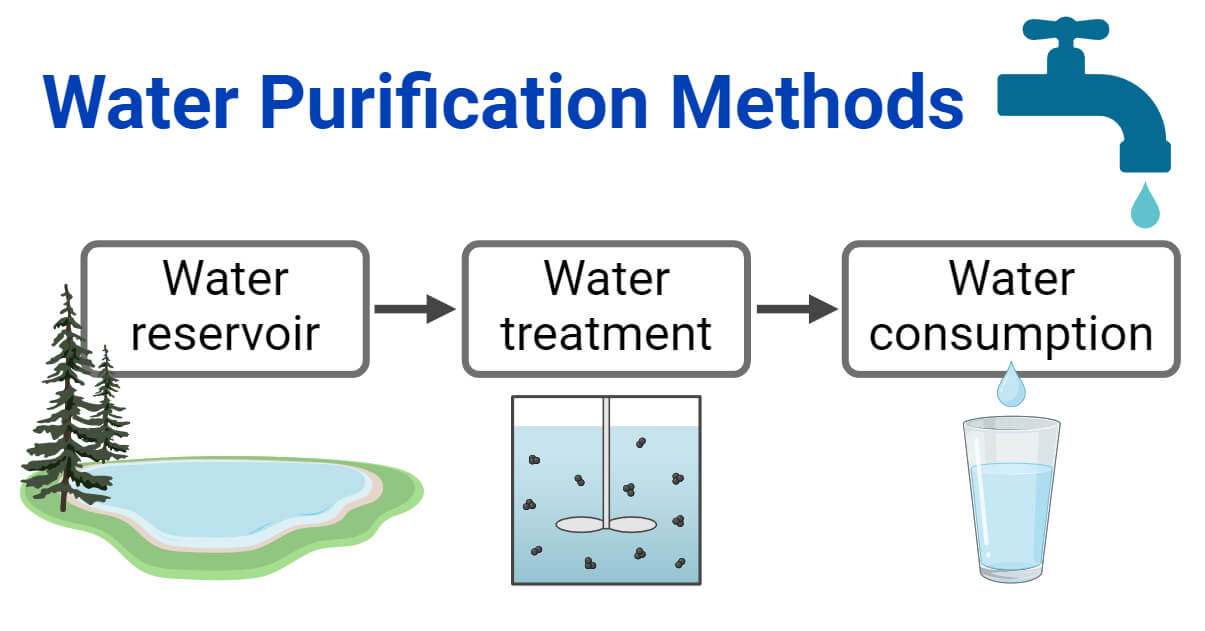
What is Water Purification?
Water purification is the process of removable of impurities, microorganisms (bacteria, algae, viruses, fungi), Parasites (Giardia, Cryptosporidium, etc.), minerals (toxic metals like lead, copper, iron, nitrate, arsenic, manganese), and contaminants from raw water.
- The consumption of untreated water (including heavy metals, dirt, and microorganisms) and contaminated water is pernicious to our health and may cause numerous health problems.
- At the end of a treatment process, a small amount of disinfectant remains to reduce the risk of contamination again during distribution.
- The purified water can produce drinking water fit for human consumption or industrial use. So, treating water is necessary to obtain clean, pure, and free from disease-causing microbes water. Moreover, It improves the taste, smell, and appearance of water.
Water Purification Method
The methods of water purification are following as:
A. Natural Methods
- Aeration
- Sedimentation
- Sunlight
- Dilution
- Oxidation
- Plants and Animals (Aquatic)
B. Artificial Methods
It consists of two methods: Purification of water on a Large Scale and Purification of water on a small scale.
Purification of water on a large scale
- The main aim of this purification is to purify water clean and safe. Its treatment depends on the type and the desired standard quality of water.
- Groundwater (wells & springs) may need no treatment other than disinfection. In addition, water purification by adding Bleaching powder/Chlorinated Lime can be used as it is Cheap, Easy to use, Reliable, and safe.
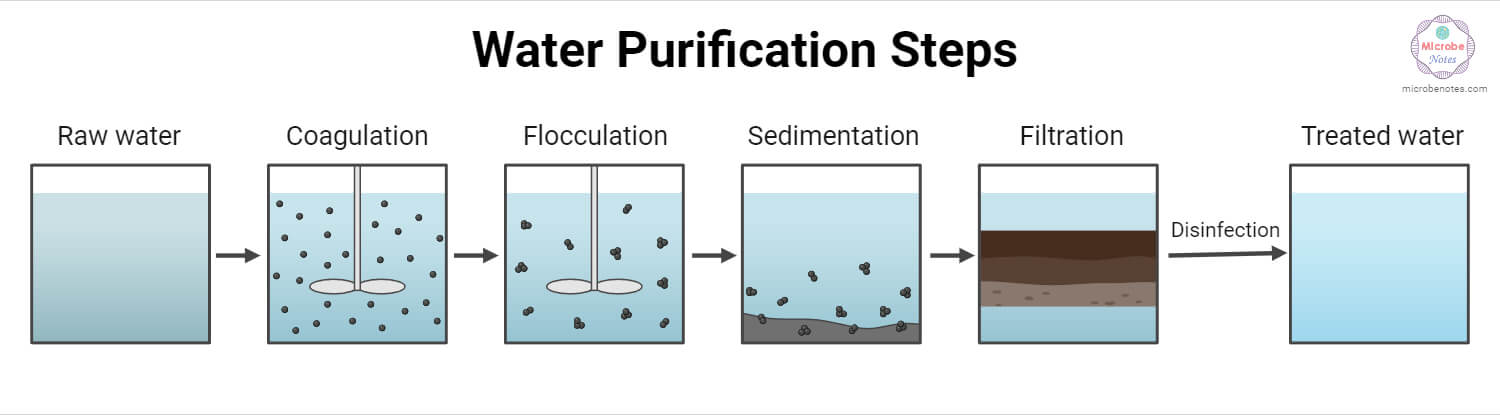
- Surface water (including Rivers, Streams, Lakes, and Reservoirs water), which tends to be turbid & polluted, requires extensive treatment. The purification follows the steps: Coagulation, Sedimentation, Filtration, and Disinfection.
The methods for water purification on a large scale are:
a. Storage
- Water is collected from the source and stored in natural or artificial reservoirs. Storage provides a water reserve that keeps pollution out.
- A significant quantity of purification takes place in storage. The optimum period of storage is 10-14 days.
As it is a natural purification, it takes place in three ways:
i. Physical:
- The water quality improves or gets better by storage. Gravity pulls about 90% of suspended contaminants to the bottom.
- It helps to provide clean water and also maintain the turbidity of water. This method permits light to pass through and lessens the work of filters.
ii. Chemical:
- During storage, some chemical changes occur. With the help of dissolved oxygen, the aerobic bacteria oxidize the organic matter in the water.
- As a result, there is a reduction in free ammonia content and a rise in nitrates.
iii. Biological:
- Due to antibiosis and oxidation, there is a large amount of reduction in bacterial count. At this time, pathogenic organisms gradually die.
- In the first 5-7 days after storing river water, the total bacterial count might decrease by up to 90 % in the first 5-7 days, which is one of the crucial benefits of preserving.
- The optimum period for storing river water is about 10-14 days. However, Long-term water storage increases the chance of developing vegetative growths like algae, which give the water a poor odor and color.
b. Filtration
- It is the most ancient and widely used purifying technique. It is the second stage in the water purification.
- Water passes through filters, from which it makes a layer of sand and charcoal that helps to eliminate smaller particles.
- The bacterial content drops by 98- 99%, turbidity by 50 PPM to 5 PPM, and color to colorless.
There are two types of filters:
i. Slow sand filtration (Biological filter):
- They are inexpensive, easy to design, and require less space. In 1804, slow sand filters were first applied to treat water in Scotland and later in London.
- Their usage is expanding all across the world. They are still widely recognized as the approved standard procedure for purifying water.
- The filter bed occupies a large area. The rate of filtration is about 100- 400 L/m2/hr. The sand granular size (diameter mm) is 0.2-0.3.
- The pretreatment of water includes sedimentation and then filter cleaning by scraping. It does not require prior water storage.
Mechanism of slow sand filter:
- Sedimentation: The supernatant water serves as a settling reservoir. Particles that can settle sink to the sand surface.
- Mechanical straining: The particles retain that cannot pass through the interstices between the sand grains.
- Adhesion: The suspended particles that settle on the sand grains’ surface are hold by adhesion to the biological layer.
- Biochemical processes in the biological layer: It eliminates organic matter, holds back bacteria, and oxidizes ammonical nitrogen into nitrates. It converts soluble iron and manganese compounds into insoluble hydroxides that adhere to the surface of the sand.
Advantages of slow sand filter
- It is simple to construct and easy to operate.
- Construction is less expensive than a rapid sand filter.
- The physical, chemical, and bacteriological quality is very high.
- The removable of bacteria is about 99.8-99.9%. E. coli drops by 99.9% and the overall bacterial count by 99.99 %.
ii. Rapid sand filtration (Mechanical filter):
- The rapid sand filter was first installed in the USA in 1885. Since then, its popularity has been in the higher remark even in developing countries.
- The rate of filtration is about 4000- 7500 L/m2/hr. The sand granular size (diameter mm) is 0.4-0.7.
- The pretreatment of water includes coagulation and sedimentation. The backwashing helps to clean the filter.
- It requires prior water storage. The removable of bacteria is about 98-99%.
- They are of 2 types: Gravity type (e.g., Paterson’s Filter) and Pressure type (Candy’s Filter).
Rapid sand filtration purifies water in the following five steps:
i. Coagulation:
- Iron or aluminum salts, such as polymers, aluminum sulfate, ferric sulfate, or ferric chloride, are added to the water during the coagulation process.
- These substances, which have a positive charge, are known as coagulants.
- Coagulation eliminates impurities and other particles present in water. Firstly, the raw water is treated with a chemical coagulant such as alum (5-40 mg/L).
- Depending upon the turbidity and color, the doses of alum range from 5-40 mg or more per liter.
- Alum and other chemicals are violently mixed in water to form tiny sticky particles known as “floc” that attract dirt particles.
ii. Rapid mixing:
- After the treatment, the water is violently mixed for a few minutes in a “mixing chamber”.
- It allows the alum to completely disperse throughout the bulk of water, which is essential.
iii. Flocculation:
- The treated water is slowly and gently stirred in a flocculation chamber for about 30 minutes by paddles.
- This type of filter contains a number of paddles that rotate at 2 to 4 rpm with the help of motors.
- As a result, it forms a thick, profuse, white floc precipitate of aluminum hydroxide that entangles all the particulate, suspended matter with bacteria. The setting velocity increases with the precipitate thickness or flock diameter.
iv. Sedimentation:
- The coagulated water is transferred to sedimentation tanks, where it is held for two to six hours until bacteria and impurities settle down in the tank by gravity and the flocculent precipitates together.
- The combined weight of dirt and the floc become heavy and sink to the bottom of the tank during sedimentation.
- This settle down process is called sedimentation.
- It is necessary to remove at least 95% of the flocculent precipitate before adding water to the rapid sand filters.
- The sediment, also known as sludge, that collects at the bottom of the tank should be removed periodically without interfering with its functionality/ operation of the tank.
- It is necessary to clean the sedimentation tank; otherwise, molluscs and sponges may grow.
v. Filtration:
- Treated water goes into a filtration process. It is a slow sand/ biological filter.
- The different elements of slow sand filters are pretreatment, filter box (includes supernatant water, sand bed, and under drainage system), and filter control valves.
- The alum floc, which isnot able to eliminate by sedimentation, retains on the sand bed. It creates a slimy layer in slow sand filters, similar to the Zoogleal layer.
- It purifies the water by absorbing or holding back microorganisms from it. Oxidation of organic matter such as, ammonia occurs as water passes through the filters.
- Bacteria and suspended particles block the filters as filtration proceeds.
- The filters quickly get dirty and start to lose their effectiveness. When the flocculent layer becomes too thick, air bubbles or water aids in backwashing.
Advantage of rapid sand filter
- It uses raw water directly.
- Rapid sand filters do not need preliminary storage.
- The filter bed occupies minimum space.
- It will become cost-effective in the future, although it is expensive.
- Filtration is quick, 40 to 50 times more rapid than slow sand filters.
- The filters are easy to cleanse.
- The Functionality/operation is more flexible.
c. Disinfection/Chlorination
The criteria set for disinfection standards are:
- It should destroy the pathogenic microorganisms without changing the water properties, such as pH, temperature, etc. at a specific time.
- It should not be harmful and change the color.
- It should be consumable.
- It should be less expensive and easy to use.
- A residual concentration should be left there to preserve from recontamination.
- It should detect rapidly and by simple techniques in small concentration ranges.
i. Chlorination:
- On large-scale water purification, chlorine is used for disinfection, either as Chlorine gas, Chloramines, or Perchloron.
- Amidst all, Chlorine gas is preferred because it is less expensive, more efficient, quick in action, and easy to use; however, it is pernicious to the eye and poisonous.
- Paterson’s chloronome is an instrument used to measure, control, and administer chlorine gas to water.
- Chlorination is one of the best methods of water purification. Although it kills pathogenic bacteria present in water, it does not affect spores and some viruses.
- It oxidizes manganese, hydrogen sulfide, and iron. The taste and odor are also improved since it aids in the destruction of some odor-producing components.
- It reduces the growth of algae. It controls the coagulation of acid and slime organisms. Moreover, It maintains residual disinfection.
Principle:
- Water should be clear and turbidity-free for the chlorination treatment. Chlorination is not as effective when water has turbidity.
- It is necessary to estimate the quantity of chlorine added. To eradicate bacteria and viruses, free chlorine must be present for at least 1 hour of contact.
- It does not affect spores, protozoal cysts, or helminthic ova, except in higher doses.
- The minimum recommended concentration for free chlorine is 0.5 mg/Liter for one hour.
- During storage & distribution, The free residual chlorine provides a margin of safety against microbial contamination.
The different tests to measure residual chlorine are the Orthotolidine test (OT) and Orthotolidine Arsenite (OTA) test.
Advantages of chlorination:
- It is less expensive.
- Ease of application
- It kills almost all bacterial contaminants.
Disadvantage of chlorination:
- It forms halogenated compounds that are carcinogenic.
ii. Ozonation:
- It is a virucidal and powerful oxidizing agent, which was used before in Europe and Canada. It has no residual effect and must be used with chlorination.
iii. Other agents:
a. UV rays:
- One of the disinfection methods is UV rays, which was used in the UK.
- It is an expensive method. The water should be clear.
- It has no residual effect.
- It does not kill bacteria/germs when the water passes through the pipes that connect the treatment plant to tap.
b. Chloramine:
- The combination of chlorine and ammonia forms chloramine.
- It is less effective than chlorine.
iv. Membrane processes:
- Membrane-processes water treatment techniques are required to be promising options for reliable drinking water production.
- It is of two types: High-pressure processes and Lower-pressure processes.
a. High-pressure processes:
It consists of reverse osmosis and nanofiltration.
i. Reverse osmosis:
- It is one of the most expensive and advanced desalination systems.
- Reverse osmosis water treatment is the water treatment process of applying pressure to a saltwater solution and forcing it through a semi-permeable membrane, which lets the solvent (water) pass through but not the solute (dissolved salts).
- The solvent moves through the membrane from the higher salt concentration to the lower salt concentration.
- Reverse osmosis has a pore size of less than 0.002 μm. It does not allow monovalent ions and organics that have a molecular weight greater than 50 daltons.
- Desalination of brackish and seawater is a water treatment process that converts brackish or seawater into potable water to supply communities.
ii. Nanofiltration:
- Because of their comparatively loose structure, NF membranes can produce water more quickly using less energy.
- It allows monovalent ions such as sodium or potassium to pass through but does not allow a high proportion of divalent ions such as calcium and magnesium.
- It has a pore size of around 0.001-0.01 μm with a corresponding molecular weight cut-off ranging from 100 to 1000 Da.
- These characteristics allow NF membranes to partially remove dissolved ions while effectively removing microorganisms, organics, colloidal particles, and suspended solids even though the many components are identified as target pollutants for drinking water treatment.
Advantages:
i. Easy automation and a compact size
ii. Broad-spectrum elimination of different water impurities to ensure excellent water quality
iii. It can adjust different feed water quality.
iv. It is effective for removing color-forming organic compounds.
b. Low-pressure processes:
It is of two types: Ultrafiltration and Microfiltration
i. Ultrafiltration:
- It does not allow organic molecules to have a molecular weight greater than 800 daltons. It has a pore size of 0.002-0.03 μm.
ii. Microfiltration:
- It can remove particles greater than 0.05 μm. It has a pore size of 0.01-12 μm.
- It is used to treat water combined with coagulation.
Purification of water on a small scale
It is carried out by:
a. Household purification
i. Boiling:
- Boiling for the purification of water is satisfactory for household purposes.
- Boiling upto 10-20 minutes kills almost all organisms, such as bacteria, spores, cysts, and ova, and removes temporary hardness by removing carbon dioxide and precipitating the calcium carbonate.
- After boiling, the taste of water alters, but it gets sterilized and harmless.
- It is one of the excellent techniques to clean water, but it does not provide any long-term defense against microbial pollution contamination.
- To prevent contamination during storage, keep the sterilized water in the same container where it boiled.
ii. Chemical disinfection:
a. Bleaching powder:
- Bleaching powder, also known as chlorinated lime (CaOCl₂), is a white amorphous powder with a pungent smell of chlorine but an unstable compound.
- It is cheap, easy to use, reliable and safe. Freshly prepared bleaching powder contains 33 % of “available chlorine”.
- It loses its chlorine content quickly when exposed to air, light, and moisture.
- It is also known as “stabilized bleach” because it retains its strength when mixed with lime.
- Bleaching should be kept in a closed container, maintaining a dark, cool, and dry place.
- A 5% solution can be used.
- Dose: 3-6 drops/L contact time of ½ hour.
b. Chlorine solution:
- Bleaching powder is used to make chlorine solutions.
- A 5 % chlorine solution can be prepared by mixing 4 kg of bleaching powder with 25% available chlorine.
- One drop of this solution will be added to 1 L of water to disinfect the water.
- The market offers ready-made chlorine solutions in a variety of strengths.
- It loses its chlorine content when exposed to air, light, and moisture or might be on prolonged storage.
c. Chlorine tablet:
- Halazone Tablets are available in the market. They work well for disinfecting small quantities of water.
- One tablet (0.5 g) will disinfect 20 litres of water.
d. High test hypochlorite (HTH)/ Perchloron:
- This calcium compound, High strength Ca-Hypochlorite, contains 60–70% of available Cl.
- One gram will disinfect one liter of water.
- It is more stable than bleaching powder and deteriorates less during storage.
e. Iodine:
- One of the treatments used for the disinfection of water is iodine.
- 2 drops of 2% ethanol solution of iodine will be sufficient for one liter of water (2 drops of 2% Soln./liter).
- It is effective for disinfection when it takes 20 to 30 minutes of contact time.
- It is unlikely to be used widely as a disinfectant for municipal water supplies.
- Its main drawbacks are its high cost and physiological activity related to thyroid activity.
f. Potassium Permanganate:
- It is a powerful oxidizing agent; however, it is ineffective as a water disinfectant.
- It is no longer recommended for disinfection of water in recent times. It has less effect on many organisms, although it kills Vibrio cholera.
- The other drawback of potassium permanganate is it alters water’s color, smell, and taste. An amount of it gives just pink coloration to the Water.
iii. Filtration:
- Small-scale water is purified by filtration through ceramic filters such as Pasteur Chamberland filter, Berkefeld Filter & Katadyn Filter.
- The Pasteur Chamberland filter consists of porcelain candles, whereas the Berkefeld Filter consists of infusorial candles.
- The essential part of a filter is Filter candles made up of porcelain or infusorial earth, having the potential to accumulate bacteria and impurities.
- It can eliminate bacteria found in drinking water; however, viruses that pass through the filter are not.
b. Disinfection of well
- In rural areas, wells are the main source of water supply.
- It needs to disinfect wells during epidemics of cholera, gastroenteritis, etc.
i. Adding bleaching powder:
- The most effective and less expensive method of disinfecting wells.
- A 2.5 g bleaching powder is used to disinfect 1000 liters of water. After one hour of contact period, water should used for drinking.
- It is good to use water in the morning for drinking if it disinfects the well at night.
References
- https://pubs.acs.org/doi/pdf/10.1021/acsestwater.3c00301
- https://www.slideshare.net/WASSAN14CH18/water-purification-methods-61592227
- https://www.slideshare.net/SurajDhara2/water-purification-140399512#59
- https://www.safewater.org/fact-sheets-1/2017/1/23/conventional-water-treatment
- https://www.slideshare.net/racinrush/water-purification-47412261
- https://www.slideshare.net/MYSTUDENTSUPPORTSYST/water-purification-on-small-scale-in-english-250452236
- https://www.slideshare.net/RizwanSa/water-purification-large-scale
- https://www.acciona.com/water-treatment/desalination/?_adin=12033057740
- Guo, H., Li, X., Yang, W. et al. Nanofiltration for drinking water treatment: a review. Front. Chem. Sci. Eng. 16, 681–698 (2022). https://doi.org/10.1007/s11705-021-2103-5
- https://www.slideshare.net/sanjaygeorge90/purification-of-water-community-medicine
- https://www.slideshare.net/kuldeepvyas370/water-purification-84899137
- https://www.thewatertreatments.com/water-treatment-filtration/rapid-sand-filters/
- https://www.cdc.gov/healthywater/drinking/public/water_treatment.html
