Intricate pathways that govern and sustain diverse metabolic activities in our bodies are abundant in the field of biochemistry. The uronic acid route is one such mechanism that is essential for the metabolism of carbohydrates. We shall examine the specifics of this route in this article, including its importance, important enzymes, steps, regulation, related illnesses, and prospective diagnostic and therapeutic uses.
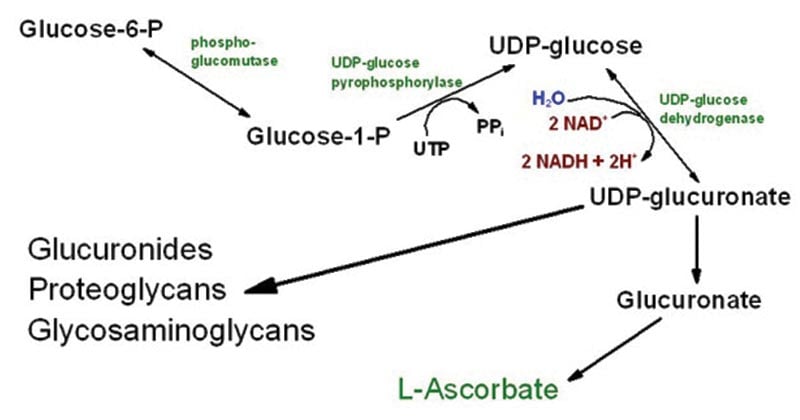
Understanding the metabolic pathways that power crucial functions becomes crucial as we work to fathom the complexity of our biological systems. The metabolism of carbohydrates is aided by the complex network of enzyme processes known as the uronic acid pathway. This pathway is crucial for preserving the structural stability of extracellular matrices and controlling a number of physiological processes.
Interesting Science Videos
What is the Uronic Acid Pathway?
Through a series of enzymatic processes known as the uronic acid pathway, certain carbohydrates like galactose and glucose are transformed into matching uronic acid derivatives.
- Uronic acids are important constituents of glycosaminoglycans (GAGs), proteoglycans, and other physiologically relevant compounds. These acids include glucuronic acid and galacturonic acid.
- The basic metabolic process present in living things is the uronic acid pathway, sometimes referred to as the uronate pathway or the hexuronic acid pathway.
- It is essential for converting carbohydrates like glucose and other sugars into uronic acids, which are necessary intermediates in a number of metabolic processes.
Synthesis of Ascorbic Acid
- Ascorbic acid (vitamin C) production plays a crucial role in the uronic acid pathway.
- Ribulose-5-phosphate is changed into UDP-glucuronic acid, which is used as a starting material for the production of ascorbic acid.
- The enzyme gluconolactone oxidase then converts UDP-glucuronic acid into L-gluconolactone.
- L-gluconolactone then goes through several processes to create ascorbic acid, which satisfies the body’s need for this crucial vitamin.
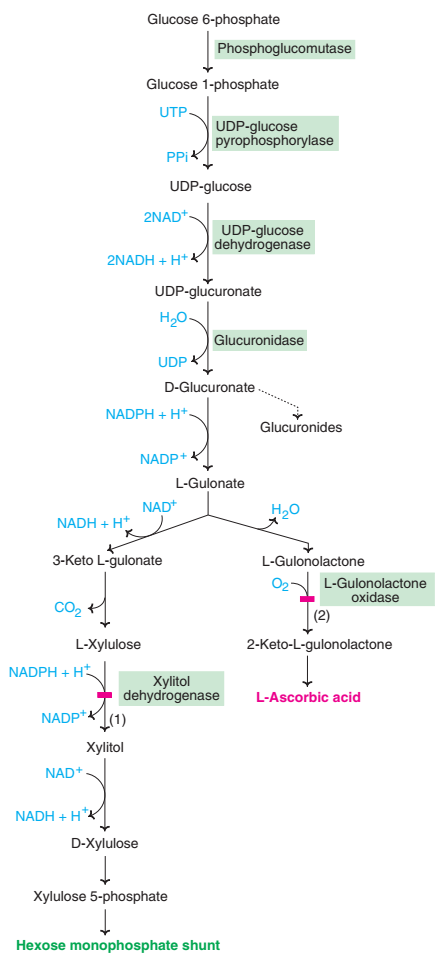
Fate of L-Gulonic Acid
Cellular metabolism may use L-gluconic acid, the direct precursor of ascorbic acid, in a variety of ways. Ascorbic acid, which is necessary for many physiological activities, may be produced using it. Alternately, further metabolism of L-gluconic acid can result in the creation of other uronic acids needed for particular metabolic processes, such as the synthesis of glycosaminoglycans.
Reaction And Key Enzymes
- Enzymatic modifications, oxidation-reduction processes, and interconversion of diverse sugar intermediates are all part of the reactions in the uronic acid route.
- Hexokinase, glucose-6-phosphate dehydrogenase, gluconolactonase, and 6-phosphogluconate dehydrogenase are just a few of the enzymes that are essential for catalyzing these processes and converting glucose into uronic acids effectively.
- Several key enzymes are involved in catalyzing the reactions within the uronic acid pathway.
- These enzymes include glucose and galactose dehydrogenases, glucuronyl transferases, and glucuronidases.
- Each enzyme plays a distinct role in converting sugars into their respective uronic acids or vice versa.
Steps of the Uronic Acid Pathway
The uronic acid pathway comprises a series of enzymatic steps, each leading to the conversion of specific sugars into uronic acid derivatives. These steps involve oxidative reactions, isomerization, and epimerization processes. Let’s explore the major steps of the uronic acid pathway:
- Step 1: Sugar Oxidation – In the initial step, glucose or galactose is oxidized by the respective dehydrogenase enzyme, resulting in the formation of glucuronic acid or galacturonic acid.
- Step 2: Epimerization – Epimerization reactions occur, interconverting glucuronic acid and galacturonic acid through the action of epimerases. This step allows for the generation of diverse uronic acid derivatives.
- Step 3: Uridine Diphosphate (UDP) Activation – The uronic acids are activated by the attachment of a uridine diphosphate (UDP) moiety, catalyzed by UDP-glucose dehydrogenase or UDP-galactose 4-epimerase.
- Step 4: Glycosylation – In this step, glucuronic acid or galacturonic acid is transferred onto specific acceptor molecules, such as proteins or GAGs, by the action of glucuronyl transferases.
Regulation of the Uronic Acid Pathway
The uronic acid pathway is tightly regulated to maintain homeostasis and ensure the proper functioning of metabolic processes. The activity of key enzymes within the pathway is modulated by various factors, including substrate availability, hormonal signals, and gene expression regulation.
Conversion in Animals
In animals, uronic acids have crucial roles in various physiological processes. They contribute to the formation of glycosaminoglycans, which are essential components of connective tissues. Uronic acids are also involved in the synthesis of certain polysaccharides and serve as precursors for the production of ascorbic acid, an essential nutrient for numerous biological functions.
Oxidation of Drugs
- Certain medicines and xenobiotics in the body are oxidized through the uronic acid pathway.
- Drugs are metabolized and changed into more water-soluble chemicals by enzyme activities within the route, which speeds up the process of eliminating them from the body.
- This procedure, referred to as drug oxidation, is crucial to the metabolism and detoxification of drugs.
- The conversion of glucose and other carbohydrates into uronic acids occurs via the intricate and crucial uronic acid route.
- It is essential for the formation of glycosaminoglycans, ascorbic acid synthesis, and drug metabolism.
- Our knowledge of basic biochemical processes is improved by a better understanding of the uronic acid route, which also advances medical research, medication discovery, and the treatment of metabolic diseases.
Effect of Drugs on Uronic Acid Pathway
Certain drugs can influence the uronic acid pathway. For example, some medications may inhibit specific enzymes involved in the pathway, leading to altered uronic acid metabolism. Such effects can have implications for drug metabolism, the synthesis of essential molecules, and overall cellular function.
Disorders Associated with the Uronic Acid Pathway
Dysregulation or genetic defects within the uronic acid pathway can lead to various disorders. Examples include mucopolysaccharidoses (MPS) and hereditary inclusion body myopathy (HIBM). These conditions are characterized by impaired GAG metabolism and can result in severe developmental abnormalities and tissue dysfunction.
Essential Pentosuria
- Essential pentosuria is a metabolic disorder that affects the uronic acid pathway.
- It is characterized by the excretion of excessive amounts of L-xylulose, a sugar intermediate produced during the pathway.
- This condition arises due to a deficiency of the enzyme L-xylulose reductase.
- While essential pentosuria is generally considered a benign disorder, understanding its impact on the uronic acid pathway contributes to our knowledge of metabolic pathways and their regulation.
Diagnostic and Therapeutic Applications
Understanding the uronic acid pathway has paved the way for diagnostic and therapeutic advancements. Measurement of urinary or plasma uronic acid levels can serve as a diagnostic tool for certain metabolic disorders. Moreover, targeted interventions and therapeutic approaches aimed at restoring or modulating uronic acid metabolism hold promise for the treatment of associated diseases.
Conclusion
The production of necessary macromolecules and control of carbohydrate metabolism are both accomplished through the uronic acid pathway, which is a crucial metabolic mechanism. Its crucial function in preserving structural integrity and detoxifying activities, as well as the complex series of enzymatic reactions it undergoes, highlights its importance. We can open up new avenues for research into human health and illness as well as new opportunities for diagnosis and therapy by deciphering the complexity of the uronic acid system.
The conversion of glucose and other carbohydrates into uronic acids occurs via the intricate and crucial uronic acid route. It is essential for the formation of glycosaminoglycans, ascorbic acid synthesis, and drug metabolism. Our knowledge of basic biochemical processes is improved by a better understanding of the uronic acid route, which also advances medical research, medication discovery, and the treatment of metabolic diseases.
References
- Zhao, Yingxin, and Allan R. Brasier. “Uronic acid pathway metabolites regulate mesenchymal transition and invasiveness in lung adenocarcinoma.” Biotarget 3 (2019).
- URONIC ACID PATHWAY (Glucuronic pathway) – http://biocheminfo.com/2020/05/14/uronic-acid-pathway-glucuronic-pathway/
- Uronic acid pathway – https://www.slideshare.net/ashokktt/uronic-acid-pathway-70775281
