The term hydrophilic means “water-loving”. The two divided parts of the hydrophilic prefix “hydro” means water, and the suffix “philic” means loving.
A hydrophilic molecule is a water-soluble molecule that can strongly interact with water through hydrogen bonding. They have positive or negative charges or partial charges. Even though hydrophilic molecules are often water-soluble, “hydrophilic” is defined independently of solubility.
- Water is a polar molecule composed of two hydrogen atoms and one oxygen atom that joins together by covalent bonds. Two atoms can form covalent bonds when they share electrons.
- Water acts as a solvent and is a hydrophilic molecule that can dissolve by interacting with the dipoles on water molecules. Hydrophilic substances can form hydrogen bonds with water.
- Polar molecules are hydrophilic and have an unequal distribution of electrons. Due to this unequal distribution of electrons, the hydrogen and oxygen atoms acquire partial positive and negative charges.
- Hydrophilic substances diffuse in water. Due to the attraction between hydrophilic molecules and water, it moves from high concentration to low concentration.
- When water moves into an area with a high concentration of molecules, it pulls the molecules apart, and it passes to a low concentration.
- Diffusion is an essential process in living beings as it helps to move the molecules in and out of the cells. Diffusion is a process of the movement of molecules from an area of higher concentration to an area of lower concentration until the concentration becomes equal throughout.
Interesting Science Videos
Chemistry behind hydrophilicity
- The “hydrophilicity” word refers to the degree or extent to which a molecule or surface attracts water.
- The most common hydrophilic functional groups are -OH, -COO-, -NH-, -Aln (OH)m, etc. The polarity of hydrophilic molecules determines their hydrophilicity.
- According to the functional group and hydrogen bonding capacity, any surface’s hydrophilicity changes: non-polar < polar, no hydrogen bonding < polar, hydrogen bonding < hydroxylic, ionic.
- The main parameter to determine the degree of hydrophilicity is contact angle measurement, which also measures the degree of wettability.
- The liquid’s capacity to remain in contact with a solid surface is known as wettability. The angle formed between the surface and the edge of the droplet is known as contact angle (θ).
- Good wettability is a characteristic of hydrophilic substances. A hydrophilic surface has a contact angle (θ) <90° depending upon the water droplet contact angle. A liquid disperses across a surface, covering a substantial area, if the contact angle is smaller than 90°.
Examples of Hydrophilic
- Enzymes
- Sugar or Glucose
- Cell membranes
- Keratin
- Salt
- Wool
- Cotton
- Sponge
- Lithosphere
- Latex Paint
- Konjac flour
- Citric acid
- Metamucil
- Talc
- Silica
- Gypsum
- Polyethylene glycol ethers
- Polyacrylic amide
- Polyurethanes with polyethylene glycol ether
- Polyvinyl alcohol (PVA)
- Polysaccharides (such as cellulose) and their derivatives (such as hydroxypropylmethylcellulose, hydroxyethylcellulose, and sodium carboxymethylcellulose)
- Gelatin, agar, agarose, algin
- Alcohols
- Cyclodextrins
- Glucon-D
- poly-N-vinylpyrrolidone (PVP)
- Guar gum, xanthan gum
- Pectin
- Xanthum
- Vegetable oil
- Calcium Carbonate
- Limestone
- Dextran
- Carrageenan
- Inulin
- Chitosan
- Egg Albumin
Enzymes
- Enzymes are the catalysts for biological reactions that occur in living organisms. Enzymes can be large or small, weakly acidic or basic, hydrophilic or hydrophobic, positively or negatively charged, or neutral.
- Since enzymes are proteins, the specific sequence of amino acid residues (Side chains or R groups) within the active sites forms a protein. When proteins have the proper structure to take up a substrate and lower the activation energy of a chemical reaction, they become functional enzymes.
- The outside of the protein must be hydrophilic to distribute and move around the cell since water is the solvent in all cell cytosol.
- Most proteins have a hydrophilic property that enables them to fill cells and generate large quantities of necessary products for the body.
Cell Membranes
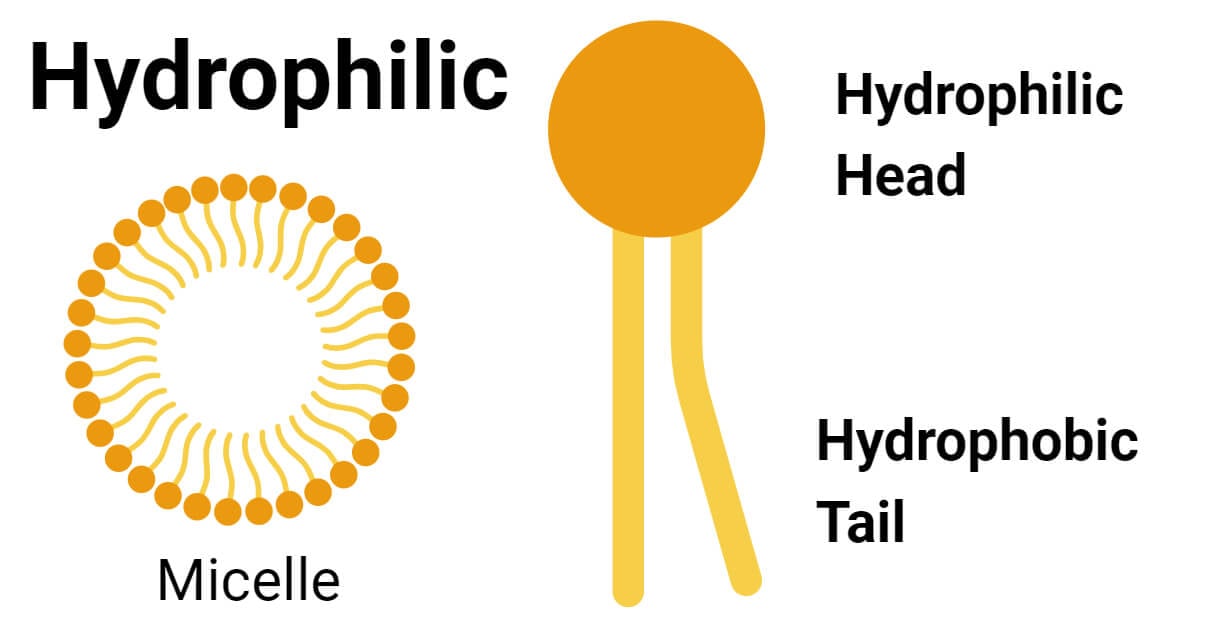
- Cell membranes form from two sheets of molecules known as phospholipids, which are amphiphilic.
- The head of a phospholipid molecule is the hydrophilic region. The outermost part of the protein is hydrophilic, which is exposed to the environment and the cytoplasm.
Glucose
- Glucose is a source of energy for many types of cells. Water can interact with electric dipoles and dissolve glucose.
- As energy is stored in the glucose bond, every cell uses glucose to power cellular functions.
- In the human body, glucose is transported from the gut to the liver into the bloodstream. It transports to all the body parts because glucose is a partially hydrophilic molecule.
Hydrophilic Surfaces
- Hydrophilic surfaces are hydrophilic coatings that do not react with water. The examples are Lithosphere (water absorbed in pores present in the cell), Latex Paint (water excreted by the latex print layer and does not evaporate from the surface), Concrete (water absorbed by the concrete mixture), Granite layer (water penetrates within the granite).
Hydrophilic Colloids
- A hydrophilic colloid, or hydrocolloid, is a colloid system in which the colloid particles are hydrophilic polymers, dissolved in water, and can spread throughout water. It can be either irreversible or reversible.
- Hydrocolloids can have a variety of forms, such as gel, aqueous, or solid, depending upon the amount of water available.
They are classified into four groups according to their origin and way of manufacturing:
- Hydrocolloids obtained from plants without any chemical modification
- Hydrocolloids produced by fermentation
- Hydrocolloids obtained from plants with chemical modification
- Hydrocolloids derived from animals
The naturally occurring vegetable hydrocolloids are classified according to their botanical origin and plant organism’s function:
- Exudates (protective colloids deposits on wounds): acacia gum/gum Arabic, tragacanth, karaya gum, ghatti gum;
- Seed flours (reserve polysaccharides): guar gum, locust bean gum, tara gum, tamarind seed gum;
- Extracts from land plants and marine algae (scaffolding substances): pectins, agar, alginate, carrageenan, starches, cellulose, furcelleran, and larch gum.
The examples are:
- Metamucil (soluble fiber having low saturated fats),
- Konjac flour(water-soluble fiber),
- Starch (water-soluble colloid obtained from maize),
- Egg Albumin (water-soluble at room temperature),
- Gelatin (obtained by hydrolysis of proteins from cows and fish),
- Pectin (extracted from citrus peel and apple pomace).
Hydrophilic emulsifiers: Hydrophilic emulsifiers act as emulsifying agents that depend upon water molecules. Examples are Xanthum, Cream and ointment, Sodium Laurel Sulfate, Soap, Vegetable oil, and Glycerol.
Hydrophilic polymers: They are water vapor permeable due to ionic groups such as acid, a form of salt like a sulfonate, ammonium, or carboxylate group, or hydrophilic elements like ethoxy ethers or amide segments. Examples are Ethylene Glycol, Ethanol, Glutamic Acid, Polyacrylamide (hydrophilic polymer with a linear structure of hydrocarbons having one nitrogen atom), Polyethyleneimine, and poly-(hydroxyethyl methacrylamide).
Hydrophilic amino acids: They form a group of hydrocarbon structures and other atoms. Examples are Glutamine, Lysine, Histidine, Arginine, Asparagine, Threonine.
Hydrophilic molecules: They attract and dissolve with water molecules. Examples are Calcium Carbonate, Chalk, Limestone, Sodium Chloride, and Glucon-D.
Applications of Hydrophilic
- Hydrophilic emulsifiers are widely used as a thickening agent (Xanthum) and emulsifying agent (Glycerol). Sodium Laurel Sulfate is used in toothpaste and cleaners. Starches are used as thickening and gelling agents in foods. In dairy products like yogurt and pudding, it gives proper texture. It provides jelly consistency in moulded products, the right gel texture, and stretches to chewy candies.
- In the fields of physics, chemistry, engineering, biomedical, drug delivery, food, pharmaceuticals, paint, textiles, paper, construction, adhesives, coatings, water treatment, dispersing and suspending agents, stabilizers, thickeners, gellants, flocculants and coagulants, film-formers, humectants, binders and lubricants, personal care, building products, detergents, oil field products, and mineral processing, etc., Hydrophilic polymers and molecules are used.
- Hydrophilic coatings are widely used in biomedical fields. The main advantage of using hydrophilic coatings is that they provide better lubricity. It plays a crucial role in the cleaning up of oil spills.
- Hydrophilic polymers like Cellulose, Alginate, and chitosan thickening agents, stabilizers, and gelling agents are used in the food industry. Carboxymethyl cellulose and polyvinyl alcohol are used as thickening agents in ice cream.
- Polyethyleneimine, or natural polymers (e.g., histone, collagen), are also used as chemical reagents to transfect nucleic acids into cultured mammalian cells.
- Polyethyleneimine is used as a detergent and as a water treatment agent.
- Polyurethane (PU) sponges, naturally hydrophilic, are used to adsorb organic solvents from water surfaces due to their high porosity and low density, resulting in a high adsorption ability at low costs.
- Hydrocolloids are obtained from algae that include agar, carrageenan, and alginate.
- Pectin acts as a gelling, thickening, and stabilizing agent in foods. It is used in pharmaceuticals. High methoxyl (HM) Pectin helps to control moisture and gives structure, bite, and bake stability to acidic jams, jellies, and confectionaries with high sugar content. It helps to improve volume, retain moisture and softness, and freeze stability in bread and frozen dough. Low methoxyl (LM) pectin helps to thicken fruit-based ripples and toppings with pumpable consistency. LM pectin used as a fat substitute in sauces, dressing, ice cream, processed meat, and cheese products, and spreads in low-fat products.
- Carrageenans are used in dairy goods such desserts, puddings, ice cream, mousses, whipped creams, spreads made of cheese, cream cheese, neutral milk drinks (such as calcium-enriched milk, nutritional beverages, chocolate milk, and milkshakes), and water-based jellies as thickening and gelling agents. It is used in soups, sauces, and salad dressings. It is used in meat products such as cooked ham. It stabilizes the injected water.
- Alginates act as emulsifiers, gelling agents, coating agents, and thickeners in the food industry. It is used in diet and light products, baked goods, frozen foods, mayonnaise, salad dressings, dessert jellies, ice cream, mousses, foams, processed cheese, meat and canned vegetables, and soups.
- Latex paint additives help to improve hydrophilic stains like household stains such as sauces, tea, coffee, red wine, colored markers, etc. The washability of hydrophilic stains relies on the water resistance of the film.
- Hydrophilic molecules are used in the food industry, and for daily purposes. Sodium chloride is a salt and dissolves in water.
- Hydrophilic amino acids are found in green vegetables, eggs, fish, dairy products, potatoes, seafood, etc.
- Glutamine, N-rich amino acid, is found in green vegetables.
- Lysine is present in eggs, potatoes, fish, milk products, etc.
- Histidine is obtained from wheat, rice, dairy food, etc.
- Asparagine is found in seafood, dairy products, legumes, etc.
- Threonine is derived from poultry, fish, lentils, cheese, etc.
- Konjac glucomannan, a non-ionic polysaccharide hydrophilic colloid, is widely used as a food additive in drinks and foods. It acts as a stabilizer and thickener in the food industry, biopharmaceutical industry, and fields associated with human health and agriculture. It increases the swelling power and water-holding capacity of the KGM–starch mixtures because of their potent hydrophilic capacity. It also enhances freeze-thaw stability and paste clarity of wheat starch. It improves the functional properties of starch.
- Dextran, a highly hydrophilic and biocompatible natural polysaccharide, is widely used for therapeutic purposes, and dextran-based hydrogels are used in biotechnological applications such as drug delivery of hydrophilic drugs.
- Hydrophilic materials play a crucial role in oil-water separation in environmental and energy aspects.
- Hydrophilic molecules
- Calcium Carbonate – It reacts with water. It produces effervescence of hydrogen gas.
- Chalk – Due to the composition of calcium carbonate, it absorbs water.
- Sodium Chloride (salt) – It readily dissolves in water.
- Limestone – It absorbs water and dissolves when pieces of limestone are mixed in water.
- Glucon-D – It is a simple sugar that mixes with water.
- Commercial hydrophilic membranes are thin, unsupported, highly porous films that filter aqueous and organic solvents used in HPLC and offer maximum chemical and pH resistance.
- A hydrophilic syringe filter helps to remove particles from an aqueous sample. It also purifies and sterilizes aqueous solutions so that they do not damage equipment. It also helps to remove bacteria from the solution.
References
- https://biologydictionary.net/hydrophilic/
- https://byjus.com/biology/diffusion/
- https://chemistry.stackexchange.com/questions/44646/hydrophilic-vs-water-soluble
- https://www.biologyonline.com/dictionary/hydrophilic
- https://open.lib.umn.edu/humanbiology/chapter/4-6-enzymes/
- https://www.khanacademy.org/science/ap-biology/cellular-energetics/enzyme-structure-and-catalysis/a/enzymes-and-the-active-site
- https://www.collegesidekick.com/study-guides/introchem/hydrophilic-and-hydrophobic-colloids
- https://link.springer.com/chapter/10.1007/978-3-642-59978-1_18
- https://application.wiley-vch.de/books/sample/352733758X_c01.pdf
- https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.1598
- https://pubs.rsc.org/en/content/articlehtml/2010/sm/b914166a
- https://coatings.specialchem.com/tech-library/article/latex-paint-additives-improve-washability-hydrophobic-hydrophilic-stains
