Interesting Science Videos
What is Nucleoside?
A nucleoside is a molecule with a pentose sugar linked to a nitrogenous base or glycosylamines. A nucleoside can also be defined as a nucleotide without a phosphate group attached to it.
- The nucleosides present in DNA contain a 2` – deoxy – D- ribose sugar and nucleosides in RNA contain D-ribose sugar.
- The main difference is seen at the second position of the pentose structure, in the case of 2` – deoxy -ribose there is an absence of an alcohol group/ oxy group/ -OH group at the second position, hence the name.
- In the case of D – ribose pentose the –OH group is present at the second position.
- In both types, the pentoses are present in their β-furanose form which is a close five-membered ring structure.
Types of Nucleoside
The nucleosides can be divided into two types based on the presence of the nitrogen base of the compound.
- Purine nucleosides
- Pyrimidine nucleosides
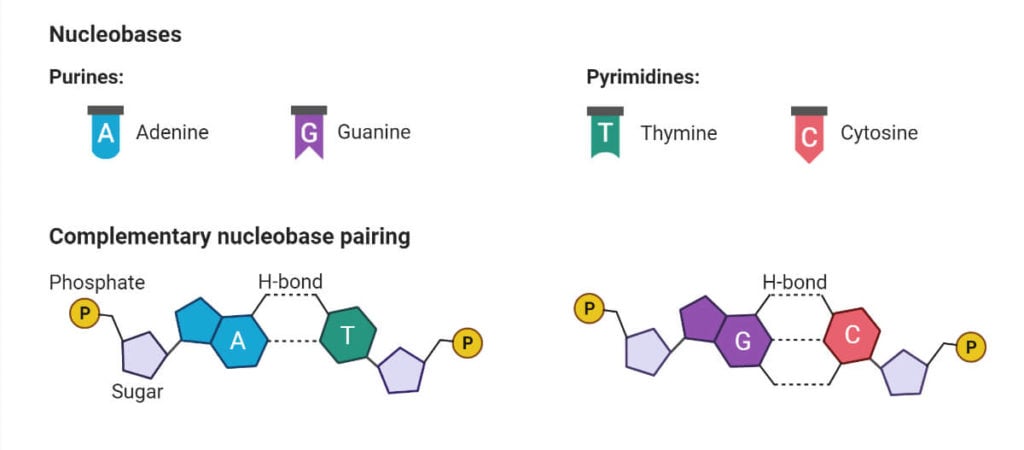
Purine nucleosides
These nucleosides are composed of two nitrogenous bases known as Adenine and Guanine.
In the case of RNA, the nucleosides are – Adenine and Guanine
In the case of DNA, the nucleosides are – Deoxyadenosine and Deoxyguanosine
Pyrimidine nucleosides
These nucleotides are composed of three nitrogenous bases known as Thymine, Cytosine, and Uracil.
In the case of RNA, the nucleosides are – Cytidine and Uridine
In the case of DNA, the nucleosides are – Deoxycytidine and Thymidine or Deoxythymidine
Structure of Nucleoside
The nucleosides consist of two main heterocyclic components –
Pentose sugar
It is a five-membered ring structure generally described as a puckered conformation.
- In the case of DNA, the nucleosides lack –OH group at the second position of pentose ring and hence is called as 2` – deoxy – D – ribose.
- In RNA it is a D – ribose pentose ring structure.
- The pentoses are present in β- furanose form in both types of sugars.
- The pentose sugar is attached to the nitrogenous base at the primary carbon atom (1`).
- It is linked by a glycosidic bond (N-β-glycosyl bond).
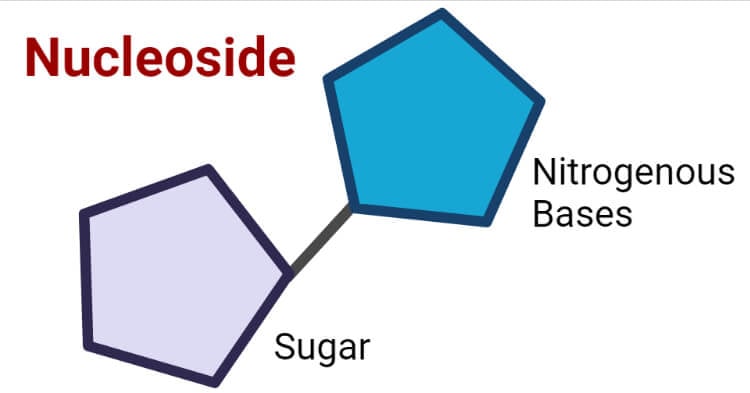
Nitrogenous base
It is a cyclic carbon structure containing nitrogen and has the properties of a base.
- The bases are derivatives of two major parent compounds – Purines and Pyrimidines.
- In RNA the nitrogenous bases are – Adenine, Uracil, Cytosine, and Guanine.
- In DNA the nitrogenous bases are – Adenine, Thymine, Cytosine, and Guanine.
- The bases are joined covalently to the pentose sugar by N-β-glycosyl bond.
- In the case of purines the N-9 atom bonds to the pentose sugar.
- In the case of pyrimidines the N-1 atom bonds to the pentose sugar.
- Although the majority of the bases are derived from purines and pyrimidines, some minor bases are also present in the DNA.
- Mostly these minor bases occur in methylated forms of purines and pyrimidines.
- In the case of some viral DNA, the bases might be hydroxyl-methylated or they might be glycosylated.
- Some of the unusual bases found in DNA are 5-Methlycytidine, N6-Methlyadenosine, 7-Methlyguanosine, 4-Thiouridine.
Functions of Nucleosides
- Nucleosides are an integral part of nucleotides. They are precursors for nucleotides. When a phosphate group is attached to the nucleoside it forms the nucleotide which is the backbone of DNA.
- Nucleoside molecules also function as signaling molecules.
- Minor bases or altered nitrogen bases in nucleosides have a role in regulating or protecting genetic information.
- Many nucleoside analogs have been used in the treatment of malignancies, tumors, and viral infections. This is achieved by some modifications in the purine and pyrimidine bases.
- Cytarabine (cytosine arabinoside) was the first drug approved by the US FDA for treatment against acute myeloid leukemia.
- Some nucleoside analogs are also used in treatments of Human Immunodeficiency Virus (HIV) disease. Lamivudine is being used in this treatment.
- Nucleoside transporters play a vital role in the transportation of nucleosides across membranes.
- There are two types of nucleoside transporters – Concentrative and Equilibrative. These transporters play an important role in the transport of antiviral and anticancer drugs across membranes.
References
- Mirza A. Z. (2019). Advancement in the development of heterocyclic nucleosides for the treatment of cancer – A review. Nucleosides, nucleotides & nucleic acids, 38(11), 836–857. https://doi.org/10.1080/15257770.2019.1615623
- https://www.frontiersin.org/articles/10.3389/fphar.2018.00606/full
- Lehninger Principles of Biochemistry – 6th ed- c2013.
