Interesting Science Videos
Laboratory diagnosis of Francisella tularensis
Specimen
- Scrapings from infected ulcers, lymph node biopsies, and sputum, whole blood.
- Serum is generally collected from all patients early in disease and during convalescence.
- To minimize the loss of viable organisms, samples should be transported to the laboratory within 24 hours.
- If specimens are to be held longer than 24 hours, specimens should be refrigerated in Amie’s transport medium.
- F. tularensis should remain viable for up to 7 days stored at ambient temperature in Amie’s medium.
- Specimens for molecular testing should be placed in guanidine isothiocyanate buffer.
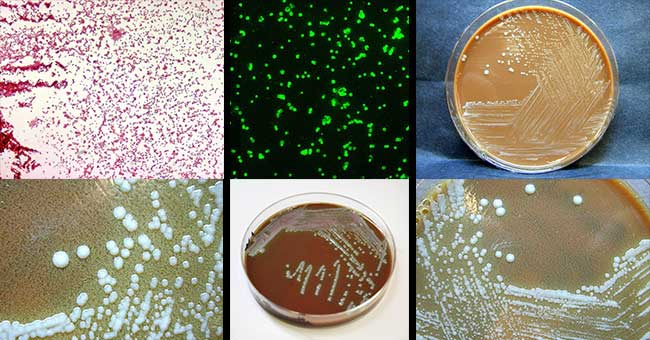
Direct detection method
- Gram staining of clinical material is of little use with primary specimens unless the concentration of organisms is high, as in swabs from wounds or ulcers, tissues, and respiratory aspirates.
- Gram negative coccobacilli are seen in Gram staining procedure.
- A more sensitive and specific approach is direct staining of the clinical specimen with fluorescein labeled antibodies directed against the organism.
- Fluorescent antibody stains and immunohistochemical stains are commercially available for direct detection of the organism in lesion smears and tissues and are typically available in reference laboratories.
Culture
- The organism is strictly aerobic and is enhanced by enriched media containing sulfhydryl compounds (cysteine, cystine, thiosulfate or IsoVitaleX) for primary isolation.
- Two commercial media for cultivation of the organism are available: glucose cystine agar and cystine-heart agar, both require the addition of 5% sheep or rabbit blood.
- However, F. tularensis can grow on chocolate agar or buffered charcoal yeast extract (BCYE) agar, media supplemented with cysteine that are used in most laboratories.
- They are slow-growing organisms and require 2 to 4 days for maximal colony formation.
- They are weakly catalase positive and oxidase negative.
- Identification is done by growth on chocolate agar but not blood agar (blood agar is not supplemented with cysteine) is also helpful.
- The identification is confirmed by demonstrating the reactivity of the bacteria with specific antiserum (i.e., agglutination of the organism with antibodies against Francisella).
Antibody detection
- Because of the risk of infection to laboratory personnel and other inherent difficulties with culture, diagnosis of tularemia is usually accomplished serologically by whole-cell agglutination (febrile agglutinins or newer enzyme-linked immunosorbent assay techniques).
- Serum antibody detection is useful for all forms of tularemia.
- After the initial specimen, a convalescent sample should be collected at 14 days and preferable up to 3 to 4 weeks after the appearance of symptoms.
- Tularemia is diagnosed in most patients by the finding of a fourfold or greater increase in the titer of antibodies during the illness or a single titer of 1:160 or greater.
- However, antibodies (including IgG, IgM, and IgA) can persist for many years, making it difficult to differentiate between past and current disease.
Molecular methods
- Conventional and real-time polymerase chain reaction (PCR) assays have been developed to detect F. tularensis directly in clinical specimens.
- Of significance, several patients with clinically suspected tularemia with negative serology and culture had detectable DNA by PCR.
Treatment of Francisella tularensis
- The organism is susceptible to aminoglycosides, and streptomycin is the drug of choice.
- Gentamicin is a possible alternative and now considered as drug of choice.
- Doxycycline and ciprofloxacin can be used to treat mild infections.
- Doxycycline and chloramphenicol also have been used, although these two agents have been associated with a higher rate of relapse after treatment.
- F. tularensis strains produce β-lactamase, which renders penicillins and cephalosporins ineffective.
- Fluoroquinolones appear promising for treatment of severe tularemia.
Prevention and control of Francisella tularensis
- Preventing bites from ticks, flies, as well as mosquitos.
- Refrain from drinking untreated water.
- Wearing protective clothing and using insect repellents reduce the risk of exposure
- If working with cultures of F. tularensis in the laboratory, wearing a gown, impermeable gloves, mask, and eye protection is must.
- Prompt removal of the tick can prevent infection.
- Persons who have a high risk of exposure (e.g., exposure to an infectious aerosol) should be treated with prophylactic antibiotics.

Good sir