Interesting Science Videos
What is Iron-Hematoxylin Staining?
This is a traditional staining technique commonly used for the detection of intestinal protozoans majorly because of its permanence and ability to demonstrate, observe, and quantify with high clarity protozoans nuclear structures. Iron hematoxylin was the stain used for most of the original morphological descriptions of intestinal protozoa found in humans.
The iron hematoxylin staining method can stain the intracellular and nuclear structure’s dark compared to the cytoplasm, for easy identification of parasite morphologies.
Iron hematoxylin staining methods can be used with either fresh specimen, or sodium acetate acetic acid formalin (SAF)-preserved, or polyvinyl-alcohol (PVA)-preserved specimens.
Principle of Iron-Hematoxylin Staining
A hematoxylin solution is made up of a combination of hematoxylin, hematein, oxihematein. Hematein acts as the mordant to produce a lake i.e it reacts with ferric ammonium sulfate to produce the ferric lake (iron-hematoxylin), a basic dye. The iron-hematoxylin compound is stable and stains sharply the structures used to identify intestinal protozoan.
Objectives of Iron-Hematoxylin Staining
- To demonstrate the presence of intestinal parasites from a given stool sample.
- To differentiate protozoal parasite morphologies.
Reagents
Procedure of Iron-Hematoxylin Staining
Preparation of solutions
- Schaudinn’s solution: Into 80ml of distilled water, add a saturated solution of Mercuric Chloride, Ethyl alcohol 20ml (95%), Acetic acid 3 mL
- Iodine solution: add 98ml of Ethyl alcohol (95%) and 2ml of Tincture of iodine (2%)
- The mordant-differentiating solution: Iron alum (ferric ammonium sulfate) 2 g, 1ml of Hydrochloric acid, distilled water 98 mL
- Hematoxylin solution: Hematoxylin 0.30 g, distilled water 100 mL
Note: Iron Hematoxylin Stain and Mordant-differentiating solutions are stable individually for 12 months when stored at the right temperature. Once the working solution is prepared, let it remains stable for 7 days before using it for staining. Filtering of the stains is recommended periodically using a filter paper to remove any debris that may be present and always keep seal the container to avoid evaporation.
Preparation of PVA-samples
- PVA-preserved samples should be allowed to fix for at least 30 min and mix the content with two applicator sticks.
- Pour some of the above PVA mixtures to a paper towel and allow to stand for 2-3 minutes for effective absorption of PVA.
- Apply some of the stool samples on the paper towel to the glass slides using an applicator stick and allow it to dry in an incubator at 37°C or overnight at room temperature to ensure the smear is completely dry.
- To the dry smear, add iodine-alcohol.
Preparation of SAF-samples
- A SAF-stool sample is mixed and strained through a wire gauze to a 15ml centrifuge tube and centrifuge for 1 minute at 500xg
- Decant the supernatant and centrifuged for 10min at 500xg and collect sediments of 0.5ml to 10ml. If necessary, adjust by repeating step 1 or by resuspending the sediment in saline (0.85% NaCl) and removing part of the suspension.
- Prepare a smear on a clean glass slide, from the sediments for later, by adding a drop of Mayer’s albumin on the smear. Then add a drop of the SAF-preserved sample sediments and allow them to dry at room temperature for 30 minutes before staining.
- You can (NOT MUST) also postfix with Schaudinn’s solution before staining before initiating a trichrome stain procedure with 70% alcohol rinse then adding iodine- alcohol.
- After drying, place the smear in 70% alcohol
Staining Procedure
a. Fresh samples:
- On clean microscopic glass slides, slides, prepare thin smears of the stool samples using an applicator stick and while still wet, dip the slides into Schaudinn’s solution, for at least 30 minutes. This is called the Schaudinn’s fixation
- Place the Schaudinn’s fixed smear in 70% alcohol to remove excess fixative.
- Rinse the slide in smoothly flowing tap water at least three times
- Place slide in iron hematoxylin working solution for 4 to 5 min
- Wash slides with smooth running tap water (constant stream of water into the container) for 10 min.
- Immerse the slides in 95% ethyl alcohol for 5 minutes.
- Place the slides in 100% ethanol for 5 minutes.
- Immerse the slides in two changes in xylene for 5 minutes for each change
- Add a permount (Mounting Media is formulated for mounting and storing long-term slides) to the stain and cover with a coverslip.
- Examine the smear under a microscope at 100x and also under oil immersion before reporting.
b. PVA-Preserves samples:
- On clean microscopic glass slides, slides, prepare thin smears of the stool samples using an applicator stick and while still wet, dip the slides into Schaudinn’s solution, for at least 30 minutes. This is called the Schaudinn’s fixation
- If the fresh specimen is liquid, place 3 to 4 drops of PVA on the slide, mix several drops of fecal material, spread and allow to dry overnight at room temperature, or for a few hours in an incubator at 37oc. Note: this procedure is applied only for liquid samples for uniform mixing and not semi-solid or solid stool samples.
- Proceed with the trichrome staining procedure by placing the slides in iodine-alcohol.
- Place in 70% ethanol for 5 minutes and wash in smooth running tap water for 10 minutes
- Place slide in iron hematoxylin working solution for 4 to 5 min
- Wash slides with smooth running tap water for 10 min.
- Immerse the slides in 70% ethanol for 5 minutes.
- Then immerse the slides again in 95% ethanol for another 5 minutes
- In two changes, place the slides in 100% ethanol for 5 minutes for each change.
- Immerse the slides in two changes in xylene for 5 minutes for each change
- Add a permount (Mounting Media is formulated for mounting and storing long-term slides) to the stain and cover with a coverslip.
- Examine the smear under a microscope at 100x and also under oil immersion before reporting.
c. Staining SAF preserved samples
- Prepare slides for staining using the SChaudinn solution and let it air dry for a few hours in an incubator or overnight at room temperature.
- Place the slides in 70% ethanol for 5 min and fix with SAF as described above.
- Wash slide in smoothly running tap water for 10 minutes.
- Place the slides in iron hematoxylin working solution for 4 to 5 min.
- Wash the slides in smoothly running tap water for 10 minutes.
- Place the slides in 70% ethanol for 5 min.
- Then, place the slides in 95% ethanol for 5 min.
- Followed by immersing the slides in 100% ethanol in two changes for 5 minutes for each change.
- Immerse the slide in two changes of xylene for 5 minutes for each change.
- Add a permount (Mounting Media is formulated for mounting and storing long-term slides) to the stain and cover with a coverslip.
- Examine the smear under a microscope at 100x and also under oil immersion before reporting.
Result and Interpretation of Iron-Hematoxylin Staining
Using the iron hematoxylin staining procedure, with a specimen containing microorganisms, the cytoplasm of trophozoites and cysts will stain violet. The nuclear chromatin, chromatoid bodies, erythrocytes, and bacteria stain dark purple to black.
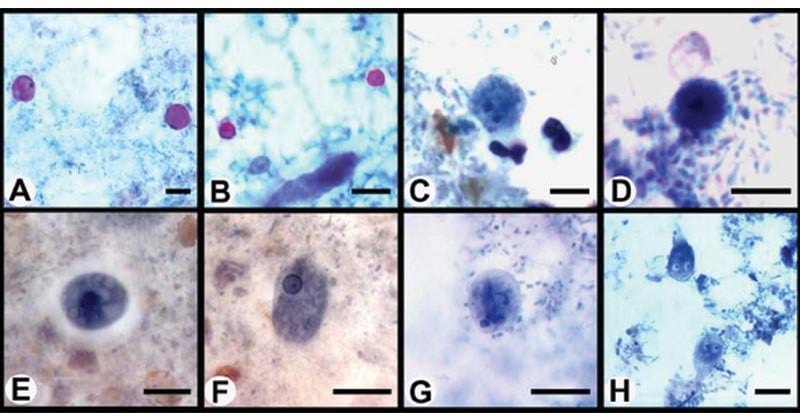
Figure: Photomicrographs of enteric protozoa stained with a modified iron-hematoxylin stain (incorporating a carbol fuschin staining step). (A) Cyclospora oocysts; (B) Cryptosporidium oocysts; (C) Dientamoeba fragilis binucleated trophozoite; (D) Dientamoeba fragilis uninucleated trophozoite; (E) Entamoeba histolytica cysts; (F) Entamoeba histolytica trophozoite; (G) Giardia cysts; (H) Giardia trophozoites. Image Source: American Society for Microbiology.
Applications of Iron-Hematoxylin Staining
- For diagnosis of parasitic infections by the demonstration of protozoal cysts and trophozoites in specimens.
- It prepares permanent stains which are important for reference.
Advantages of Iron-Hematoxylin Staining
- It is adequate for routine use in the diagnosis of intestinal protozoans.
- It offers high clarity for the differentiation of protozoans.
Limitations of Iron-Hematoxylin Staining
- If the fixation is not properly done, the result will be distorted.
- It is a slow procedure that requires adequate time to perform.
- Schaudinn solution contains mercury and therefore it must be removed completely before staining. it interferes with the staining reagents.
References and Sources
- 1% – https://www.researchgate.net/publication/26274071_Pancreatic_Remodeling_Beta-Cell_Apoptosis_Proliferation_and_Neogenesis_and_the_Measurement_of_Beta-Cell_Mass_and_of_Individual_Beta-Cell_Size
- 1% – https://www.researchgate.net/post/Does_anyone_has_done_a_very_good_luxol_fast_blue_staining_using_cryosections
- 1% – https://www.dalynn.com/dyn/ck_assets/files/tech/SI70.pdf
- 1% – https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.832.446&rep=rep1&type=pdf
- 1% – http://www.med-chem.com/para-site.php?url=procedures&subsection=microscopic&tertiary_section=permanent_stained_smear&quad_section=pss_iron_hematoxylin_stain
- <1% – https://www.jove.com/t/3954/rat-mesentery-exteriorization-model-for-investigating-cellular
- <1% – https://www.cdc.gov/meningitis/lab-manual/chpt06-culture-id.html
- <1% – https://www.cdc.gov/dpdx/diagnosticprocedures/stool/staining.html
- <1% – https://www.abcam.com/ps/pdf/protocols/ihc_p.pdf
- <1% – https://science.howstuffworks.com/engineering/structural/water-slide4.htm
- <1% – https://microbeonline.com/types-of-staining-techniques-used-in-microbiology-and-their-applications/
- <1% – https://eduwavepool.unizwa.edu.om/lmsdatapool/00011253/LearningObjects/Parasitology%20Practical%20Manual.doc
- <1% – https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Basic_Principles_of_Organic_Chemistry_(Roberts_and_Caserio)/22%3A_Arenes%2C_Electrophilic_Aromatic_Substitution/22.10%3A_Oxidation_Reactions
- <1% – http://www.atlas-protozoa.com/microscope-exam-ed.php
- <1% – http://shs-manual.ucsc.edu/policy/fecal-microscopic-examination

Please I need the references. It is very important
References are there at the end of the note.