The human papillomavirus represents a group of viruses that is diagnosed in more than 90% of cervical cancers and is the most common pathogen responsible for female cancers.
There are more than 100 subtypes of HPV. It is mainly associated with multiple epithelial lesions and cancers, predominantly on cutaneous and mucosal surfaces.
It is the most common sexually transmitted disease in the world that significantly impacts the social life of the individual.
HPV is classified into two categories: low-risk HPVs (LR-HPVs) that cause anogenital and cutaneous warts, and high-risk HPVs (HR-HPVs) that cause oropharyngeal cancers and anogenital cancers including cervical, anal, vulvar, vaginal, and penile cancers.
HPV itself does not cause cancer but is triggered through various other factors like smoking, exposure to UV and other carcinogenic chemicals, folate deficiency, immunosuppression, and pregnancy.
Interesting Science Videos
Structure of Human Papillomavirus (HPV)
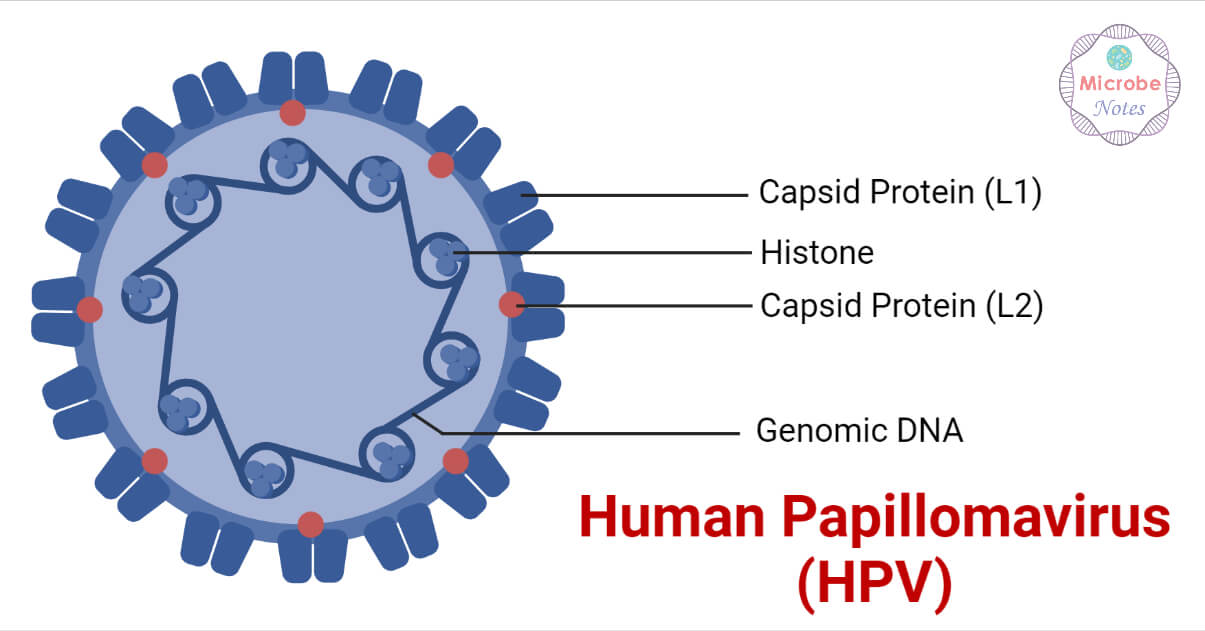
- The papillomaviruses are small, non-enveloped and 52-55nm in diameter having icosahedral symmetry.
- It consists of a single double-stranded DNA enclosed in a protein capsid composed of 72 pentameric capsomers.
- The capsid is made up of two structural proteins, which include the L1 (Late 1) and L2 (Late 2) viral proteins.
- The L1 is 55kDa in size and makes up 80% of the viral proteins. The L2 is 70kDa in size. Both the L1 and L2 proteins are virally encoded.
- The viral coat contains 360 molecules of L1 protein arranged into 72 capsomers and a variable number of L2 molecules, which are not fully exposed on the surface of the virion.
- The expression of L1 either alone or in combination produces Virus-like particles (VLPs) in mammals or non-mammals.
- The virion density is 1.34 g/ml in cesium chloride with a sedimentation coefficient (S20, W) of 300.
Genome Structure of Human Papillomavirus (HPV)
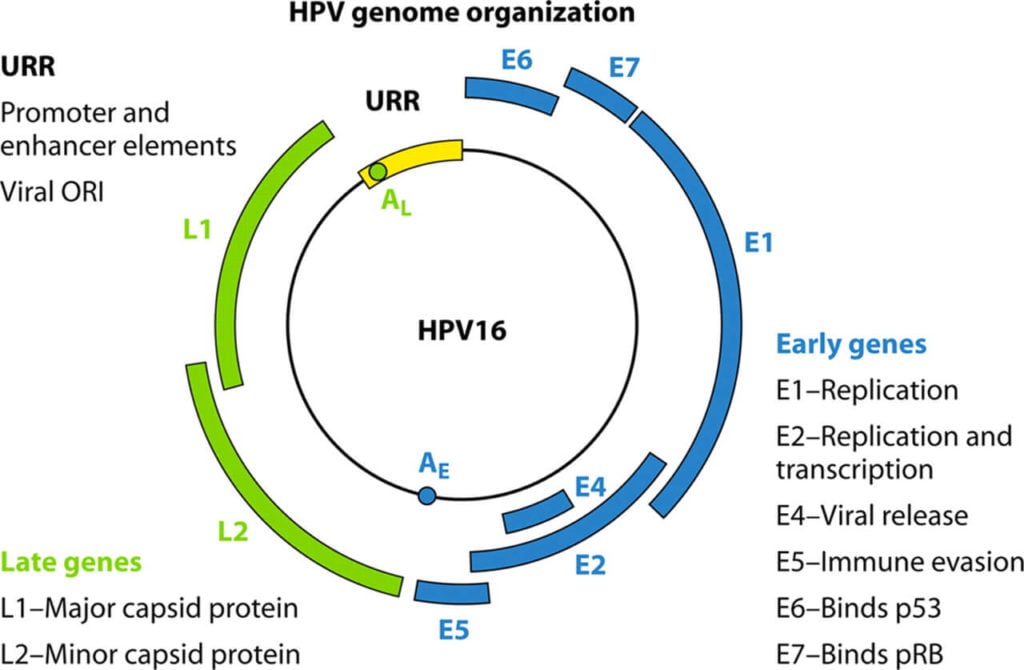
- The genome of all HPV types contains approximately eight ORFs transcribed into a single double-stranded DNA molecule of about 800 bp genome size.
- The ORF can be divided into three functional regions, which include the early region (E), the late region (L), and a large non-coding part referred to as the long control region (LCR).
- The E region encodes proteins (E1-E7) that are required for replication, the L region codes for structural proteins (L2-L2) important for viral assembly, and the LCR contains the cis-elements necessary for the replication and transcription of viral DNA.
- Although the papillomaviruses differ in their size and number of ORFs, they contain well-conserved core genes that are involved in replication (E1 and E2), genes for packaging (L1 and L2) while having high diversity in the remaining genes (E6, E7, E5, and E4) that help in cell entry, immune invasion, and virus release.
Epidemiology of Human Papillomavirus (HPV)
- There are over 180 identified subtypes of HPV.
- HPV subtypes 1, 2, 4, 27, and 57 are responsible for causing cutaneous warts on hands and feet, such as verruca vulgaris or verruca plantaris. These are spread through contact between skin with epidermal damage or through infected fomites.
- HPV subtypes 6 and 11 are generally associated with anogenital warts, such as condyloma acuminatum. They are termed low-risk HPV and are also associated with juvenile and adult recurrent respiratory papillomatosis.
- Subtypes 16 and 18 most commonly cause pre-cancerous and cancerous lesions of the cervix, male and female anogenital areas, and oropharyngeal area.
- Subtypes 31, 33, 35, 45, 52, and 58 also belong to the high-risk HPV and are associated with cervical cancer development.
- Although both the low-risk and high-risk HPV are considered sexually transmitted agents, they can also transmit through other forms of close contact.
- Recent studies according to the Center for Disease Control and Prevention (CDC) show the prevalence of HPV for adults aged 18 to 59 to be approximately 45.2% in men and 39.9% in women.
- The genital HPV infection is the most common sexually transmitted infection in the United States and worldwide.
Transmission of Human Papillomavirus (HPV)
Different subtypes of HPV affect the mouth, throat, or genital area. Although it a common STD, it can be asymptomatic and resolve on its own. Penetrative sex is not required for the spread of the virus.
It can transmit through:
- Skin-to-skin contact of the genital area
- Vaginal, anal, or oral sex
- Sharing of sex toys
Some of the risk factors for acquiring HPV infection include:
- Sexual activity, age of first sexual intercourse, and number of sexual partners
- Smoking
- Use of oral contraceptives (for more than 5 years)
- Chewing betel nut
- Exposure to radiation and UV light
Replication of Human Papillomavirus (HPV)
- The HPV enters the cells through microabrasions. HR-HPV, a type of HPV causing cervical and other cancers, can enter the cell through the single-layered squamous cellular junction between the endo and ectocervix. The HPV L1 capsid protein binds to the cellular receptors like heparin sulfate proteoglycans (HSPGs) located on the basement membrane or surface of basal layer cells.
- HPV enters the cell through endocytosis which has most similarities with micropinocytosis.
- The virus travels through the membrane-bound cytoplasmic components and trans-Golgi apparatus.
- Through a tubulin-mediated pathway, the viral episomal genome then gets transported into the nucleus through the nuclear pores or by the breakdown of the nuclear membrane during mitosis.
The viral replication phases include:
Early-phase of viral replication
- The early transcription is initiated upon the nuclear entry.
- The E2 binds E1, which binds as a dimer of hexamers to the origin of the replication site and recruits cellular DNA replication machinery.
- The HPV genome generates approximately 50-100 episomal copies per nucleus in the initial replication.
Late-stage of viral replication
- It involves the replication of vegetative viral DNA and virion formation.
- This process requires increased expression of viral E1 and E2 proteins.
- It is marked by the activation of the viral major late promoter situated in the E7 gene region that not only results in the increased expression of E1 and E2 but also of E4 and E5.
- The late stage of viral replication most likely uses the rolling circle mechanism for DNA replication to produce thousands of progeny viral genomes.
- The virion formation takes place in the nucleus following the viral genome amplification and capsid protein synthesis.
- The viral maturation takes place in the superficial keratinocytes and finally leads to the release of the infectious virion.
Pathogenesis of Human Papillomavirus (HPV)
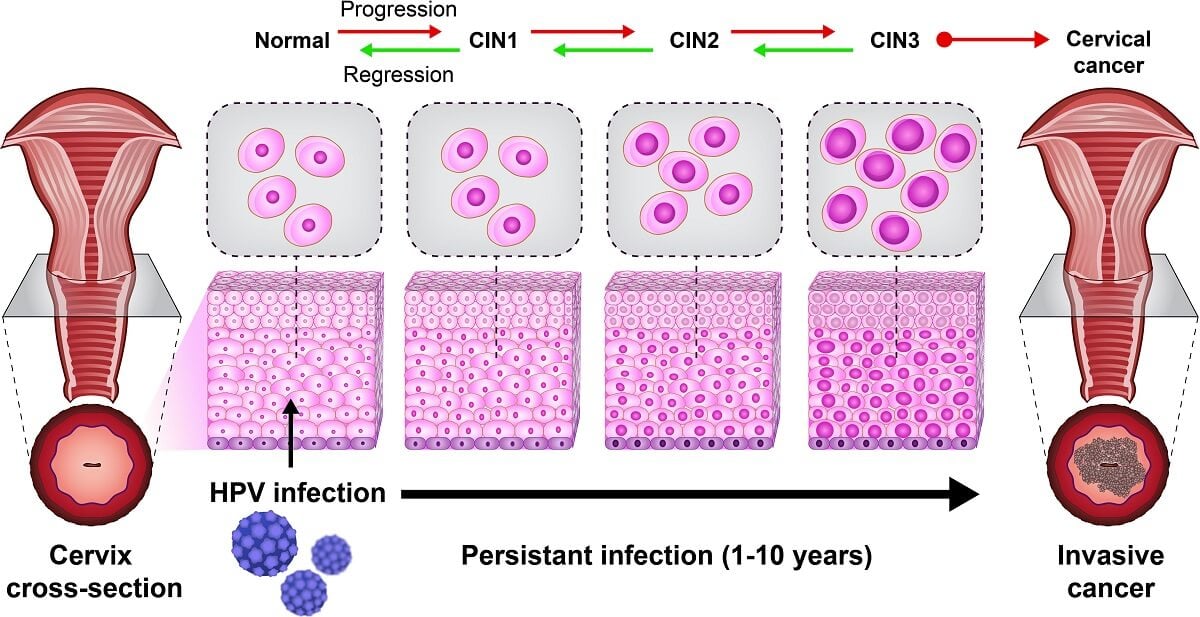
- Although having high prevalence rates, HPV infections usually resolves spontaneously within a year or two. The persistence of the infection is an important contributor to the development of cervical and other cancers.
- HPVs infect cutaneous or mucosal epithelial cells and initiate benign or cancerous lesions depending on the HPV type.
- As the host immune response mechanisms play a significant role in controlling the HPV infection, its prevalence is seen higher in immunocompromised individuals.
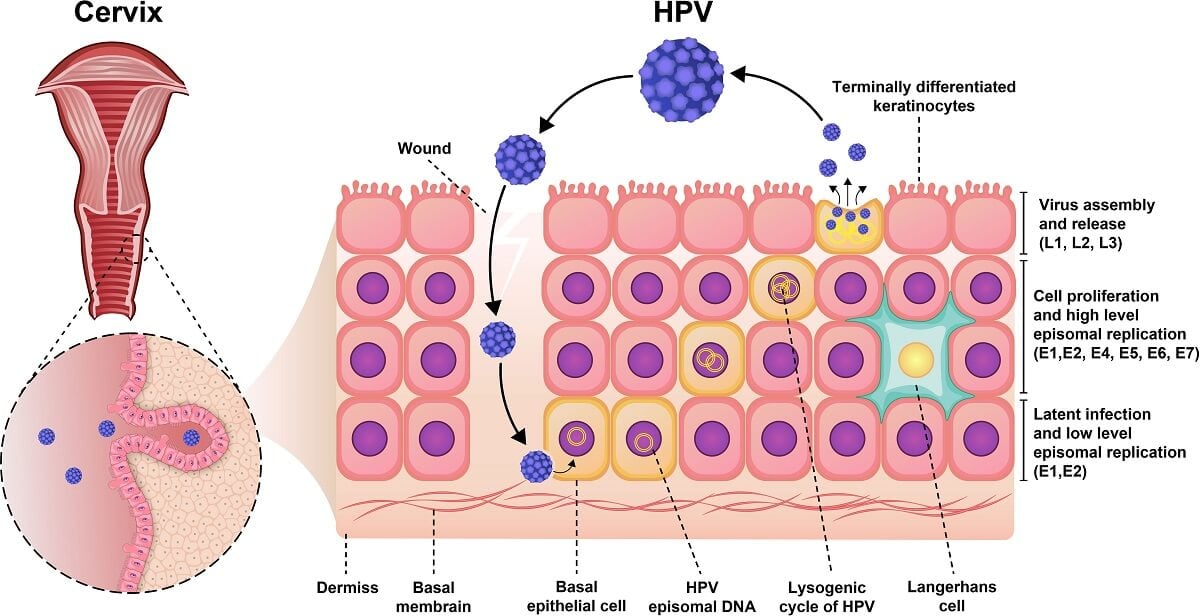
- Once the virus infects the target cells, it does not lyse the cell or cause viremia that reduces the exposure of viral antigen that aids to evade the host immune response.
- The HPV also has the ability to downregulate major histocompatibility complex class I (MHC 1) and disrupt the interferon (IFN) pathway facilitated by oncoproteins E5, E6, and E7.
- HLA molecules and intracellular viral antigenic peptides complex are transported through the Golgi apparatus and to the cell surface where they get recognized by the cytotoxic CD8+ T cells.
- The activated cytotoxic T lymphocytes (CTLs) then cause the apoptosis of the infected cells.
- The T helper cells secrete cytokines that allow proliferation, maintenance of cytotoxic lymphocytes, and activation of B cells for antibody production.
- HR-HPV types are generally associated with many types of cancers like cervical cancer and hence the presence of certain genotypes indicates a risk factor for initiation and progression of 90% of cervical cancer cases.
Clinical Manifestations of Human Papillomavirus (HPV)
Most HPV infections are asymptomatic where the infection resolves without knowledge of the patient acquiring the virus. About 90% of the patients with high-risk or low-risk HPV are cleared of the infection within two years.
The clinical symptoms of the HPV include:
Mucosal human papillomavirus infections:
- Condyloma acuminatum (Present as papules, nodules, or soft, filiform, pinkish, sessile, or pedunculated growths in the genital area)
- Focal epithelial hyperplasia (disease of the oral mucosa more common in children and women)
- Cervical neoplasia and cervical cancer
- Other anogenital cancers (including those of the vulva, vagina, penis, and anus)
- Head and neck cancer
Cutaneous human papillomavirus infections:
- Common warts
- Plantar warts
- Flat warts
- Filiform warts
- Pigmented warts
- Epidermal cysts
- Skin cancer (Bowen’s disease)
Diagnosis of Human Papillomavirus (HPV)
Traditional methods like electron microscopy, cell culture, and certain immunological testing cannot be done for HPV detection. Some important methods for its detection include:
- Warts/Condyloma detection
Visual inspection for genital or oral warts can be done by observing the pigmentation, induration, ulceration, etc. of warts and confirmed through biopsy.
- Colposcopy and acetic acid test
Colposcopy is done for the examination of the cervix, vagina, and sometimes vulva after application of acetic acid solution coupling with biopsies obtained from lesions suspected of neoplasia. The colposcopic findings are then graded according to the degree of acetowhite lesions, surface contour, mosaic pattern, and punctuation. The severity of lesions is inferred according to the abnormalities of these factors.
- Biopsy
Excisional biopsy is performed when colposcopic findings indicate high abnormalities, when it shows low-grade changes with severe dyskaryosis, or when a lesion extends to the canal.
The presence of mature squamous cells with a clear perinuclear zone known as koilocytes is the characteristic feature of genital warts.
- DNA techniques
Direct probe hybridization such as dot blot and Southern blot were initially used for HPV detection. However, they were labor-intensive, time-consuming, had low sensitivity, and required large amounts of DNA in clinical samples. The two main techniques for routine viral detection include:
- Hybrid capture HPV DNA Test 2 (hc2)
HC2 with the Pap test has been approved by the FDA. It can detect as little as 1 pg of HPV/ml. Although it cannot detect the exact HPV type, it can differentiate between the “low-risk” (6, 11, 42, 43, 44) and “high-risk” (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) genotype group.
- Polymerase chain reaction
It selectively amplifies the HPV sequences present in the biological specimens.
- Pap smear or Pap test
It was first described by Papanicolaou and Traut. It uses the scrapings from the squamocolumnar junction that is fixed on a glass slide. Malignant, premalignant changes and viral infections like HPV and Herpes infection can be detected through this method. Scrapings can be taken from the upper lateral part of the vaginal wall for cytological evaluation.
Treatment of HPV
- Various treatments can be done for cutaneous warts like surgical removal, cryotherapy, irritant or immunomodulating medications, and laser removal.
- Some pharmacological agents for treating mucosal and cutaneous warts include podophyllin resin, podophyllotoxin, organic acids, such as salicylic acid, trichloroacetic acid, and bichloroacetic acid, and cytostatic agents such as bleomycin, cidofovir, and 5-fluorouracil.
- Anogenital and oropharyngeal warts can be treated similarly in immunocompetent individuals.
- Resection, chemotherapy, and/or radiation may be required for the development of HPV-related carcinoma.
Vaccination of HPV
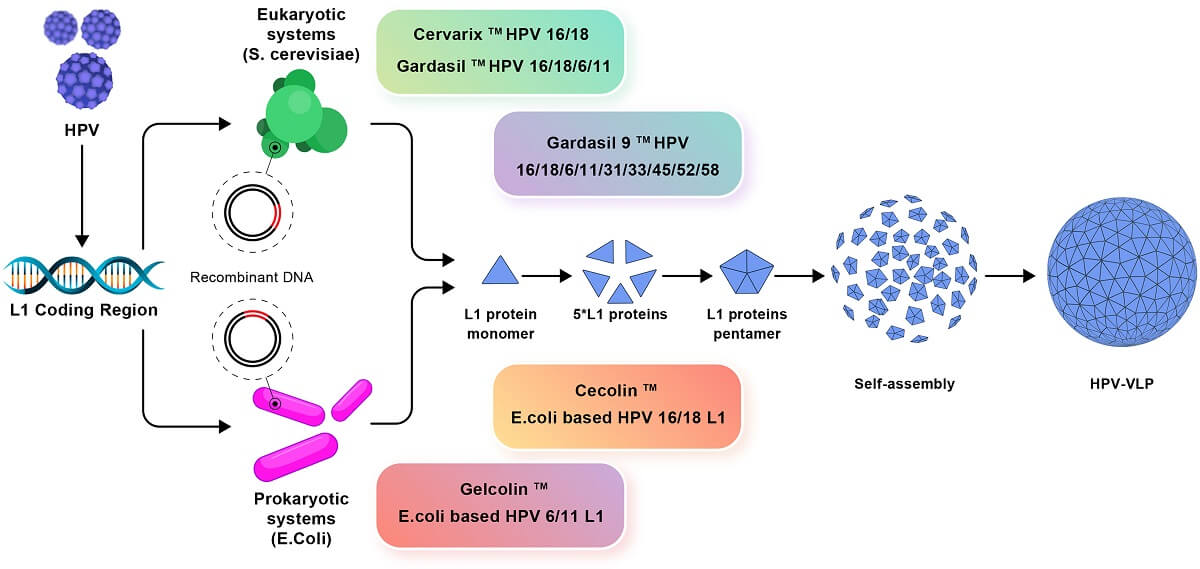
- A 9-valent recombinant protein subunit HPV vaccine is currently licensed and distributed in the United States.
- The major antigen used for the immunization against HPV is the L1 major capsid protein.
- The L1 protein is produced through recombinant technology and self-assembles into virus-like particles (VLP).
- The vaccine is administered intramuscularly.
- Routine vaccination is recommended for females and males at age 11 or 12 years and is not licensed for adults over 45 years of age.
Prevention and Control of HPV
Most people who are sexually active acquire the HPV at some point in their life, however, measures can be taken to lower the chances of getting it.
Some of the preventive interventions include:
- Vaccination should be done at 11 to 12 years of age. Vaccination through 26 years of age is recommended however, the efficacy of the vaccine decreases if administered after the onset of sexual activity.
- Use of condoms and/or dental dams during vaginal, anal, or oral sex.
- An annual vaginal cuff Pap test (BIII) should be performed for women with a history of high-grade CIN, adenocarcinoma in-suite, or invasive cervical cancer.
- Screening for lesions and treating the precancerous lesions in HIV seropositive patients can reduce the risk of anal cancer in PWH.
References
- Yousefi Z, Aria H, Ghaedrahmati F, Bakhtiari T, Azizi M, Bastan R, Hosseini R, Eskandari N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front Immunol. 2022 Jan 27;12:805695. doi: 10.3389/fimmu.2021.805695. PMID: 35154080; PMCID: PMC8828558.
- Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda G-A, Zhou Y and Jin T (2021) Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 8:552028. doi: 10.3389/fpubh.2020.552028
- Chan, C. K., Aimagambetova, G., Ukybassova, T., Kongrtay, K., & Azizan, A. (2019). Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. Journal of Oncology, 2019, 1–11. https://doi.org/10.1155/2019/3257939
- Ashrafi, G. H., & Salman, N. A. (2016). Pathogenesis of Human Papillomavirus – Immunological Responses to HPV Infection. In www.intechopen.com. IntechOpen. https://www.intechopen.com/chapters/51256
- Pinkbook | HPV | Epidemiology of Vaccine Preventable Diseases | CDC. (2020, November 2). Www.cdc.gov. https://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html
- Humans, I. W. G. on the E. of C. R. to. (2007). Human Papillomavirus (HPV) Infection. In www.ncbi.nlm.nih.gov. International Agency for Research on Cancer. https://www.ncbi.nlm.nih.gov/books/NBK321770/
- Human Papillomavirus (HPV). (n.d.). Www.who.int. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/human-papillomavirus
- Doorbar, J., Egawa, N., Griffin, H., Kranjec, C., & Murakami, I. (2015). Human papillomavirus molecular biology and disease association. Reviews in Medical Virology, 25, 2–23. https://doi.org/10.1002/rmv.1822
- Human papillomavirus (HPV). (2019, March 19). Nhs.uk. https://www.nhs.uk/conditions/human-papilloma-virus-hpv/
- Luria, L., & Cardoza-Favarato, G. (2022, January 24). Human Papillomavirus. Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK448132/?report=classic
- How Do You Prevent HPV? | Prevention Tips. (n.d.). Www.plannedparenthood.org. https://www.plannedparenthood.org/learn/stds-hiv-safer-sex/hpv/how-can-i-make-sure-i-dont-get-or-spread-hpv
- Juckett, G., & Hartman-Adams, H. (2010). Human Papillomavirus : Clinical Manifestations and Prevention. American Family Physician, 82(10), 1209–1213. https://www.aafp.org/afp/2010/1115/p1209.html
- HPV infection – Diagnosis and treatment – Mayo Clinic. (n.d.). Www.mayoclinic.org. https://www.mayoclinic.org/diseases-conditions/hpv-infection/diagnosis-treatment/drc-20351602
- Dixit, R., Bhavsar, C., & Marfatia, Y. S. (2011). Laboratory diagnosis of human papillomavirus virus infection in female genital tract. Indian Journal of Sexually Transmitted Diseases and AIDS, 32(1), 50–52. https://doi.org/10.4103/0253-7184.81257
- Human Papillomavirus Disease | NIH. (n.d.). Clinicalinfo.hiv.gov. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/human-papillomavirus-disease
