Hemes are cyclic tetrapyrroles that contain iron and are commonly found as the prosthetic group of hemoglobin, myoglobin, and cytochrome.
Heme synthesis involves a series of enzymatic reactions, collectively known as the porphyrin synthesis pathway or the heme biosynthetic pathway.
Heme synthesis is the formation of heme which begins with the condensation of glycine and succinyl-CoA to form δ-aminolevulinic acid (ALA). ALA is subsequently converted into porphobilinogen (PBG), which is further converted into protoporphyrin IX. Finally, protoporphyrin IX is combined with iron to form heme.
Interesting Science Videos
Location of Heme Synthesis and Pathway
- Heme synthesis predominantly occurs in the bone marrow, liver, and other hematopoietic tissues.
- The initial steps of heme synthesis take place in the mitochondria, while the subsequent reactions occur in the cytosol.
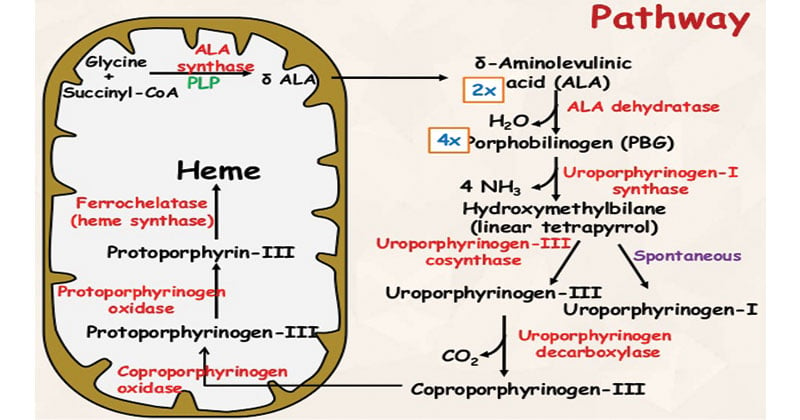
Reactions and Steps Involved in Heme Synthesis
The reactions involved in heme synthesis are as follows:
1. Condensation of Glycine and Succinyl-CoA
- In the first step of heme synthesis, the enzyme ALA synthase catalyzes the condensation reaction between glycine and succinyl-CoA.
- This results in the formation of δ-aminolevulinic acid (ALA). This reaction, catalyzed by the enzyme ALA synthase, results in the formation of δ-aminolevulinic acid (ALA).
- Reaction: Glycine + Succinyl-CoA → δ-aminolevulinic acid (ALA)
- Enzyme: ALA synthase
2. Formation of Porphobilinogen (PBG)
- The enzyme ALA dehydratase acts on two molecules of ALA, catalyzing their condensation and removal of water molecules.
- This leads to the formation of porphobilinogen (PBG).Two molecules of ALA condense to form PBG in a reaction catalyzed by ALA dehydratase.
- Reaction: 2 ALA → PBG + 2 H2O
- Enzyme: ALA dehydratase
3. Conversion of Porphobilinogen to Uroporphyrinogen III
- Porphobilinogen deaminase is the enzyme responsible for converting four molecules of PBG into hydroxymethylbilane.
- This process involves the removal of NH3 groups, resulting in the formation of uroporphyrinogen III.
- Four molecules of PBG are enzymatically converted to uroporphyrinogen III.
- Reaction: 4 PBG → Hydroxymethylbilane + 3 H2O
- Enzyme: Porphobilinogen deaminase
4. Decarboxylation of Uroporphyrinogen III
- Uroporphyrinogen III is further modified by the enzyme uroporphyrinogen decarboxylase, which catalyzes the decarboxylation of the molecule.
- This step generates coproporphyrinogen III.
- Uroporphyrinogen III is decarboxylated to form coproporphyrinogen III.
- Reaction: Hydroxymethylbilane → Uroporphyrinogen III + CO2 + H2O
- Enzyme: Uroporphyrinogen decarboxylase
5. Oxidative Decarboxylation of Coproporphyrinogen III
- Coproporphyrinogen oxidase is the enzyme responsible for the oxidative decarboxylation of coproporphyrinogen III.
- This reaction removes two carboxyl groups and converts the molecule into protoporphyrinogen IX.
- Coproporphyrinogen III is oxidatively decarboxylated to form protoporphyrinogen IX.
- Reaction: Coproporphyrinogen III → Protoporphyrinogen IX + CO2 + 2H2O
- Enzyme: Coproporphyrinogen oxidase
6. Conversion of Protoporphyrinogen IX to Protoporphyrin IX
- The enzyme protoporphyrinogen oxidase catalyzes the oxidation of protoporphyrinogen IX, leading to the formation of protoporphyrin IX.
- This step involves the removal of two hydrogen atoms.
- Protoporphyrinogen IX is oxidized to form protoporphyrin IX.
- Reaction: Protoporphyrinogen IX → Protoporphyrin IX + H2O
- Enzyme: Protoporphyrinogen oxidase
7. Insertion of Iron into Protoporphyrin IX
- The final step of heme synthesis involves the insertion of iron into protoporphyrin IX.
- The enzyme ferrochelatase facilitates the insertion of ferrous (Fe2+) iron into the protoporphyrin ring, resulting in the formation of heme.
- Iron is inserted into protoporphyrin IX, resulting in the formation of heme.
- Reaction: Protoporphyrin IX + Fe2+ → Heme
- Enzyme: Ferrochelatase
Regulation of Heme Synthesis
Heme synthesis is regulated at multiple levels to maintain the appropriate levels of heme within the body. One of the key regulatory steps is the feedback inhibition of ALA synthase by heme itself. When heme levels are sufficient, it binds to ALA synthase, inhibiting its activity and preventing the overproduction of heme.
Significance of Heme Synthesis
The creation of hemoglobin, which is in charge of delivering oxygen to red blood cells, depends on heme synthesis. Hemoglobin ensures appropriate oxygenation and cellular respiration by binding to oxygen in the lungs and releasing it to tissues all throughout the body.
Additionally, myoglobin, a protein present in muscle cells that aids in oxygen transport and storage inside muscles, requires heme as a critical component. Furthermore, several enzymes engaged in significant metabolic processes depend on heme production to operate properly.
For instance, heme-containing enzymes called cytochromes are involved in electron transport pathways. The synthesis of energy inside cells and the respiration of cells both depend on these enzymes.
Other enzymes that are involved in the defense against oxidative stress, hormone production, and nitric oxide synthesis include catalases, peroxidases, and nitric oxide synthases.
Diseases and Toxicities Associated with Heme Synthesis
- Anomalies in heme synthesis can lead to a number of diseases and toxicities.
- Porphyria, a class of uncommon genetic illnesses characterized by deficiencies in certain enzymes of the heme biosynthesis pathway, is one well-known condition.
- Abdominal discomfort, light sensitivity, neurological issues, and discolored urine are just a few of the symptoms of porphyrias.
- Examples of porphyrias linked to heme synthesis include acute intermittent porphyria and porphyria cutanea tarda (PCT).
- Heme-related toxicities can also be caused by certain medications, substances, and environmental factors. For instance, lead exposure can cause lead poisoning and interfere with heme production.
- Anemia, neurological harm, as well as other negative outcomes, can result from lead poisoning.
Conclusion
The complex mechanisms of heme production are essential for preserving the equilibrium of heme in the body. The formation of hemoglobin, myoglobin, and numerous enzymes necessary for oxygen transport, metabolism, and other vital biological processes is made possible by heme synthesis. Disorders like porphyria may result from dysregulation or anomalies in heme synthesis.
References
- David Hames and Nigel Hooper (2005). Biochemistry. Third ed. Taylor & Francis Group: New York.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- Biochemistry, Heme Synthesis – https://www.ncbi.nlm.nih.gov/books/NBK537329/
- Heme Synthesis – https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/heme-synthesis
- Yien, Yvette Y., and Mark Perfetto. “Regulation of heme synthesis by mitochondrial homeostasis proteins.” Frontiers in Cell and Developmental Biology (2022): 1167.
- Belcher, John D., et al. “Heme degradation and vascular injury.” Antioxidants & redox signaling 12.2 (2010): 233-248.
