During times of fasting or limited carbohydrate consumption, the metabolic process of gluconeogenesis is essential for keeping blood glucose levels within a normal range.
Gluconeogenesis entails the production of glucose from substances other than carbohydrates, including lactate, pyruvate, glycerol, and certain amino acids.
The primary sites for gluconeogenesis are the liver and kidneys, and it is controlled by a number of hormonal signals. The significance of this process in preserving metabolic homeostasis will be discussed after examining the precursors involved in gluconeogenesis, its location within the body, the pathway of glucose synthesis, the regulatory mechanisms involved, the impact of insulin resistance on gluconeogenesis, and other topics covered in this essay.
Interesting Science Videos
Gluconeogenesis Location
The majority of gluconeogenesis occurs in the liver, which is also where glucose is produced and delivered into the circulation. The liver has a large potential for gluconeogenesis because it contains essential enzymes that are part of the route. Although less so than the liver, the kidneys also play a role in gluconeogenesis. The need for gluconeogenesis increases after extended fasting or prolonged activity, and these organs are crucial in supplying the body’s needs for glucose.
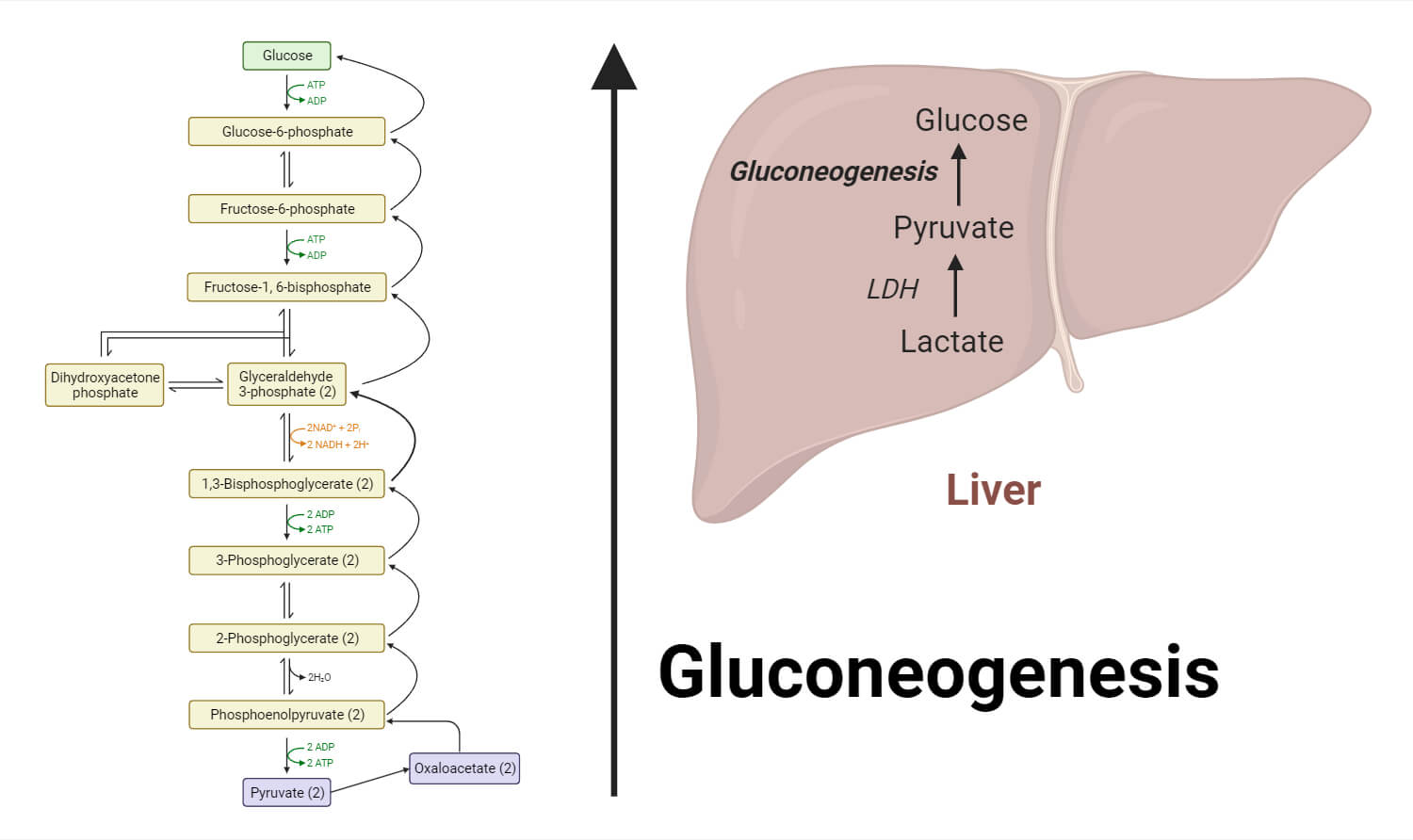
Steps of Gluconeogenesis
1 – Substrates
- Some of the many sources of the precursors for gluconeogenesis include lactate, pyruvate, glycerol, and certain amino acids.
- While glycerol is transformed to dihydroxyacetone phosphate (DHAP), a glycolysis intermediate, lactate is transferred back to pyruvate.
2 – Conversion of Pyruvate to Phosphoenolpyruvate (PEP)
- Pyruvate carboxylase converts pyruvate, which is generated from lactate or other sources, into oxaloacetate.
- ATP and biotin are needed as cofactors for this process.
- Phosphoenolpyruvate carboxykinase (PEPCK), an enzyme present in the mitochondria and cytoplasm, next transforms oxaloacetate into PEP.
3 – PEP to Fructose-1,6-bisphosphate
- A variety of enzymatic processes are used to convert PEP to fructose-1,6-bisphosphate, avoiding the irreversible events of glycolysis.
- PEP is changed by PEP carboxykinase into oxaloacetate, which is then changed into malate and expelled from the mitochondria.
- Malate is changed back into oxaloacetate in the cytoplasm, where it is phosphorylated to create PEP.
4 – Fructose-1,6-bisphosphate to Glucose
- The subsequent stages of gluconeogenesis mimic the anti-glycolytic responses.
- In order to produce fructose-6-phosphate, fructose-1,6-bisphosphatase must first remove the phosphate group from fructose-1,6-bisphosphate.
- The circulation can then receive glucose when the enzyme glucose-6-phosphatase hydrolyzes glucose-6-phosphate.
It’s crucial to remember that hormonal cues and metabolic circumstances control gluconeogenesis. Insulin inhibits gluconeogenesis, whereas hormones like glucagon, cortisol, and growth hormone encourage it. The control of these hormones contributes to the preservation of blood glucose levels within a certain range.
Precursors
- Diverse sources can yield diverse supplies of the precursors for gluconeogenesis.
- Anaerobic metabolism results in the production of lactate, which can be transformed back into pyruvate and used as a substrate for gluconeogenesis.
- Pyruvate can also be produced via the transamination of amino acids or by releasing glycerol from the breakdown of triglycerides.
- Additionally, alanine and glutamine are two amino acids that can directly support the gluconeogenic pathway.
Gluconeogenesis Pathway
- A sequence of enzyme activities that reverse many stages of glycolysis, the breakdown of glucose, make up the route of gluconeogenesis.
- Phosphoenolpyruvate (PEP) is created when pyruvate is converted into it. Oxaloacetate is created when pyruvate is carboxylated by pyruvate carboxylase.
- Phosphoenolpyruvate carboxykinase (PEPCK) then converts oxaloacetate to PEP.
- By passing the irreversible processes of glycolysis, the conversion of PEP to fructose-1,6-bisphosphate is made possible by extra enzymes.
- Finally, glucose can be released into circulation by the conversion of fructose-1,6-bisphosphate and glucose-6-phosphate by the enzymes fructose-1,6-bisphosphatase and glucose-6-phosphatase, respectively.
Gluconeogenesis Regulation
To guarantee that glucose synthesis takes place only when necessary, gluconeogenesis is strictly controlled.
- Gluconeogenesis is aided by hormones including glucagon, cortisol, and growth hormone.
- When blood sugar levels are low, the hormone glucagon is produced, which promotes the production of crucial gluconeogenic enzymes.
- Growth hormone and cortisol both promote gluconeogenesis, particularly under stressful conditions or when fasting.
- Insulin, on the other hand, prevents gluconeogenesis by reducing the expression of gluconeogenic enzymes and enhancing tissue absorption of glucose.
- The control of these hormones contributes to the maintenance of glucose homeostasis.
Insulin Resistance
The control of gluconeogenesis can be upset by insulin resistance, a disease characterized by decreased cell reactivity to insulin. When there is insulin resistance, the liver produces too much glucose because it is less susceptible to the insulin’s inhibitory effects. This is a factor in the high blood glucose levels seen in diseases like type 2 diabetes. In addition to encouraging gluconeogenesis, insulin resistance also prevents glycolysis, aggravating hyperglycemia.
Importance of Gluconeogenesis
- The gluconeogenesis cycle is crucial for controlling blood sugar levels during deprivation.
- RBCs, neurons, skeletal muscle, the medulla of the kidney, the testes, and embryonic tissue are just a few of the cells and tissues that need glucose to function.
- Metabolites including lactate, which is created by muscles and RBCs, and glycerol, which is produced by adipose tissue, are removed from the circulation through the Neoglucogenesis cycle.
Associated Disease
Deficiency in any of the gluconeogenic enzymes leads to hypoglycemia. Failure of gluconeogenesis may be fatal.
Conclusion
In conclusion, gluconeogenesis is an essential metabolic mechanism that enables the body to keep blood glucose levels steady even when there is a shortage of glucose. It includes producing glucose from non-carbohydrate precursors such as lactate, pyruvate, glycerol, and certain amino acids, and it mostly takes place in the liver and kidneys. Hormonal cues control the production of glucose; whereas insulin inhibits the route, glucagon, cortisol, and growth hormone promote it.
The control of gluconeogenesis ensures that glucose is produced only when it is required, preventing hypoglycemia or high glucose levels. But insulin resistance, a condition characterized by decreased responsiveness to insulin, can hamper this control and trigger excessive gluconeogenesis, which heightens blood sugar levels and is a factor in diseases like type 2 diabetes.
Understanding metabolic adaptations and the emergence of metabolic illnesses requires an understanding of gluconeogenesis and the mechanisms governing it. Additional investigation in this area might shed light on new treatment strategies for treating disorders linked to glucose metabolism and preserving metabolic balance.
Overall, gluconeogenesis is an intriguing process that supports good metabolic health and plays a significant part in maintaining glucose homeostasis.
References
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- Physiology, Gluconeogenesis – https://www.ncbi.nlm.nih.gov/books/NBK541119/
- Gluconeogenesis – https://byjus.com/neet/gluconeogenesis-definition/
- Gluconeogenesis – https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Metabolism/Anabolism/Gluconeogenesis
- Gluconeogenesis – https://www.britannica.com/science/gluconeogenesis

It’s absolutely amazing notes,thank you
Helpful website.
Thanks for organizing such a consice notes.
This is the most helpful site I have seen
Thank you so much
This site is very helpful to me and l want to assure all the servers of this site that l am ready to learn from from you .l use it to write my assignment and l pass them with good marks
This site is very helpful to me and l want to assure all the servers of this site that l am ready to learn from from you .l use it to write my assignment and l pass them with good marks.