Faecal specimens should be collected in the early stages of the diarrhoeal disease, when pathogens are present in the highest number, and preferably before antimicrobial treatment is started, if appropriate. The specimen should be collected in the morning to reach the laboratory before noon, so that it can be processed the same day. Formed stools should be rejected. Ideally, a fresh stool specimen is preferred to a rectal swab, but a rectal swab is acceptable if the collection cannot be made immediately or when transportation of the stool to the laboratory is delayed.
Interesting Science Videos
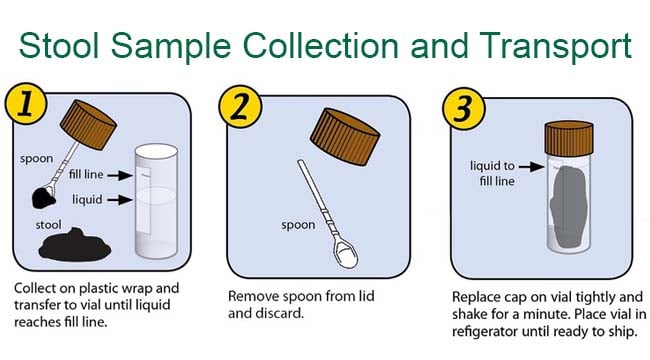
Procedure for collecting stool samples
- Provide the patient with two small wooden sticks and a suitable container with a leakproof lid (e.g. a clean glass cup, a plastic or waxed-cardboard box, or a special container with a spoon attached to the lid).
- The use of penicillin bottles, matchboxes and banana leaves should be discouraged as they expose the laboratory staff to the risk of infection.
- Instruct the patient to collect the stool specimen on a piece of toilet tissue or old newspaper and to transfer it to the container, using the two sticks.
- The specimen should contain at least 5 g of faeces and, if present, those parts that contain blood, mucus or pus. It should not be contaminated with urine.
- Once the specimen has been placed in the specimen container, the lid should be sealed.
Transport of specimen
- The patient should be asked to deliver the specimen to the clinic immediately after collection.
- If it is not possible for the specimen to be delivered to the laboratory within 2 hours of its collection, a small amount of the faecal specimen (together with mucus, blood and epithelial threads, if present) should be collected on two or three swabs and placed in a container with transport medium (Cary–Blair, Stuart or Amies) or 33 mmol/l of glycerol–phosphate buffer.
- For cholera and other Vibrio spp., alkaline peptone water is an excellent transport (and enrichment) medium.
- Pathogens may survive in such media for up to 1 week, even at room temperature, although refrigeration is preferable.
Procedure for collecting rectal swabs
- Moisten a cotton-tipped swab with sterile water. Insert the swab through the rectal sphincter, rotate, and withdraw.
- Examine the swab for faecal staining and repeat the procedure until sufficient staining is evident.
- The number of swabs to be collected will depend on the number and types of investigation required.
- Place the swab in an empty sterile tube with a cotton plug or screw-cap, if it is to be processed within 1–2 hours.
- If the swab must be kept for longer than 2 hours, place it in transport medium.

Thank you
Thanks gee
I love your blog Sagar, I teach Microbiology and I and my students find your posts very helpful
Thank you so much 🙂