Interesting Science Videos
B lymphocytes
They acquired their name from early experiments in chickens. The synthesis of antibody was shown to require the presence of an organ called the bursa of Fabricius (an outpouching of the cloacal epithelium). Surgical removal of the bursa early in life prevented antibody synthesis. Thus the cells that developed into mature, antibody-forming cells were called bursa-derived or B cells. In contrast to chickens and other birds, mammals do not have a bursa; rather, the early stages of mammalian B-cell differentiation take place predominantly in the bone marrow throughout the life of the individual.
Our understanding of B-cell differentiation has been facilitated by studies in animals in which the early embryonic stages can be easily manipulated.
For this reason, B-cell differentiation is particularly well characterized in mice and chickens, but many of these differentiation steps are common to humans as well.
- In mammals, the early stages of B-cell differentiation take place in the bone marrow and throughout the life of an individual.
- Different CD molecules are expressed at different stages of B-cell development.
- The earliest recognizable cell in the B-cell lineage is the pro-B cell, in which the first stage of Ig H-chain gene rearrangement takes place: ADH gene segment rearranges to a JH gene segment.
- The next stage is the pre-B cell, in which a VH gene segment rearranges to the joined DJ segments to form a VDJ unit, positioning the rearranged VDJ close to the Cμ gene. The pre-B cell synthesizes a μ chain that is expressed on the surface in association with non-rearranging surrogate light chains plus the signal transduction molecules Igα (CD79a) and Igβ (CD79b). The complex of μ and surrogate light chains in conjunction with Igα/β is referred to as the pre-B-cell receptor.
- In the next stage of differentiation, light-chain genes start to rearrange; surrogate light-chain synthesis is shut down, and a κ or λ chain is formed that associates with the cell’s μ chain. This cell, the immature B cell, expresses an IgM molecule in association with Igα/β on the surface of the cell. The complex of IgM and Igα/β is referred to as the B-cell receptor.
- Because the V(D)J recombination mechanism is essentially random, some immature B cells express an IgM specific for a foreign (non-self) antigen and some an IgM specific for a self-antigen. Immature B cells with receptors specific for non-self-antigens leave the bone marrow and move to the spleen. Immature B cells with receptors specific for self-antigens are deleted by apoptosis. The deletion in bone marrow of immature B cells with potential reactivity to self is an important feature of central tolerance in the B-cell lineage. Not all self-reactive immature B cells are deleted immediately. Some undergo receptor editing: V(D)J rearrangement is reactivated and the light-chain genes undergo further rearrangement. If the cell generates a receptor that is specific for a non-self molecule, the cell is “rescued” and differentiates further. If the cell generates a receptor that is still reactive to
a self-molecule, the cell is deleted. - In the next phase of B-cell differentiation, the mature B cell expresses IgM and IgD—with identical antigenic
specificity—on the cell surface. - Further development of the mature B cell occurs outside the bone marrow as a result of exposure to antigen. Activation of the B cell leads to proliferation and differentiation into plasma cells, the cells that synthesize and secrete antibody. Some activated B cells differentiate into memory cells, which make more rapid responses and synthesize non-IgM isotypes in subsequent responses to antigen.
- Thymus-dependent antigens require T-cell help to induce B-cell antibody synthesis. In the early phase
of the response, IgM is synthesized, but in the later phases of the response other isotypes—IgG, IgA, or IgE-are synthesized. - The interaction of T-helper cells and B cells takes place predominantly in the germinal centers of secondary
lymphoid organs. Events in the germinal center reaction include (a) somatic hypermutation of genes coding for antibody V regions, resulting in affinity maturation, and (b) class switch recombination, in which a B cell that was synthesizing IgM and IgD switches to synthesizing antibody of a different isotype (IgG, IgA, or IgE) with the same antigenic specificity. Cytokines synthesized by T cells influence the isotype of antibody synthesized by the B
cell. - Germinal center B cells develop into memory B cells or plasma cells. Memory cells “home” to different tissues; plasma cells home predominantly to bone marrow where they continue to synthesize antibody for a long time.
- In mucosa-associated lymphoid tissue, IgAcommitted B cells develop at an inductive site, migrate out of the lymphoid tissue and home back via the blood to a different mucosal effector site, where they complete their differentiation to IgAsecreting plasma cells.
- B-cell responses to thymus-independent antigens involve other sets of B cells—marginal-zone B cells and B-1 cells—and generate almost exclusively IgM.
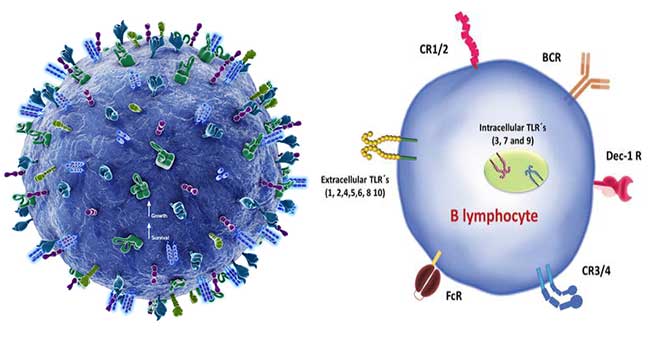
- Expression of membrane Ig is unique to B cells. CD10, CD19, CD20, and CD27 expression defines stages of B-cell differentiation. B cells also express a co-receptor, CD19/CD21/CD81/CD225, that enhances signals through the B-cell receptor and lowers the threshold for the level of antigen required to activate the B cell after binding to Ig.
- Mature and activated B cells also express an array of surface molecules that play a vital role in interactions with other cells, particularly T cells. These include MHC class II molecules, the co-stimulatory molecules B7, CD40, and ICOSL, and receptors for cytokines. B cells, like other leukocytes, express homing molecules that allow the trafficking of cells to specific tissues.

I am student in Medical laboratory….. I need more friends inoder to share knowledge….. Please search me on facebook… Musa kichuri
Email. Musakichuri@gmail.com or whatsapp +255625650835
Thank you