Interesting Science Videos
What is Auramine- Rhodamine Staining?
- The evolution in staining methodologies has led to an era of modified staining techniques that are rapid, more versatile, and reliable in result interpretation. This is one of those techniques which was modified from the Acid Fast staining technique know as Ziehl-Neelsen Staining of Mycobacterium spp of bacteria. The major difference between these two techniques is the kind of reagents used for staining and the mode of observing them.
- The auramine-rhodamine staining technique being a histological type of stain that is used to stain and demonstrate the presence of Acid Fast-bacilli under a fluorescent Microscope and also known as Truant auramine–rhodamine stain, demonstrates the anatomy of the bacterial bacilli-cell.
- Mycobacterium spp bacterial cell walls are made up of mycolic acid (Mycolic acids are long fatty acids found in the cell walls of Mycobacterium spp and they are the major and specific lipid components of the mycobacterial cell envelope and they are essential for their survival). When mycolic acid is combined with dyes they form a characteristic effect known as Acid-fastness.
- To improve the sensitivity of demonstrating Mycobacterial bacteria, Hagemann (1937) introduced the use of fluorescent dyes, and Truant, Brett and Thomas in 1962 analyzed the importance of using fluorescent dyes for acid-fast bacilli noting more yield in demonstrating high numbers of positive acid-fast bacilli. This technique was better than the Ziehl Neelsen Staining techniques.
- A combination of Auramine and rhodamine produced satisfactory results as compared to the use of the dyes separately. The complex also was found to be faster (rapid) in comparison to acid-fast staining.
- Auramine and Rhodamine are both fluorescent dyes with a high affinity for mycolic acid found on the Mycobacterium spp cell wall Hence it stains the cell wall bright yellow or orange, under a fluorescent microscope with a green background. It can also stain parasites with Sporozoa.
Principle of Auramine- Rhodamine Staining
The fluorochrome dye, Auramine-Rhodamine, used combine with the mycolic acid on the cell wall of the bacteria, which is then fixed by steamed heat. A decolorizing agent, acid alcohol is used to rinse off the none stained dyes. The counterstain Potassium Permanganate functions to stain the non-fluorescent tissues and the cell debris thus reducing possibilities of artifacts. When the cells are observed under an Ultra-violet Light, they appear bright yellow-green or red-orange.
NOTE: There is no heat fixation done for the (Primary stain) Fluorescence dyes, unlike in the Ziehl Neelsen Staining method. This is because the fluorescent dyes have a high affinity for the mycolic acid on the bacterial cell hence they bind strongly without the application of heat.
Reagents
- Auramine-Rhodamine dyes (Primary stain)
- Distilled Water
- Acid-alcohol (decolorizer)
- Potassium permanganate (counterstain)
Procedure of Auramine- Rhodamine Staining
- Prepare a thin smear of the specimen on a sterile microscopic glass slide, and gently heat fix the smear avoiding overheating.
- Add enough quantity of the Auramine-Rhodamine Dyes (Flooding) on the smear and allow it to stand for 15 minutes and ensure the dyes stain the smear well. Do not apply heat.
- Rinse the stained smear with water until no color appears in the effluent. Ensure the water is chlorine-free water possibly distilled water since chlorine interferes with fluorescence.
- Add the decolorizing agent (acid-alcohol) for 2-3 minutes to destain and wash thoroughly with distilled water and remove excess water by shaking the slide.
- Flood the smears with potassium permanganate (counterstain) for 2 minutes exactly. Note: long periods of counterstaining can quench the fluorescence of the bacilli.
- Rinse thoroughly with distilled water and allow to air dry. Do not blot.
- Examine under a fluorescent microscope at K530 excitation filter and BG12 barrier or G-362 excitation filter and LP 420 barrier or with oil immersion at 400X for verification.
Result and Interpretation of Auramine- Rhodamine Staining
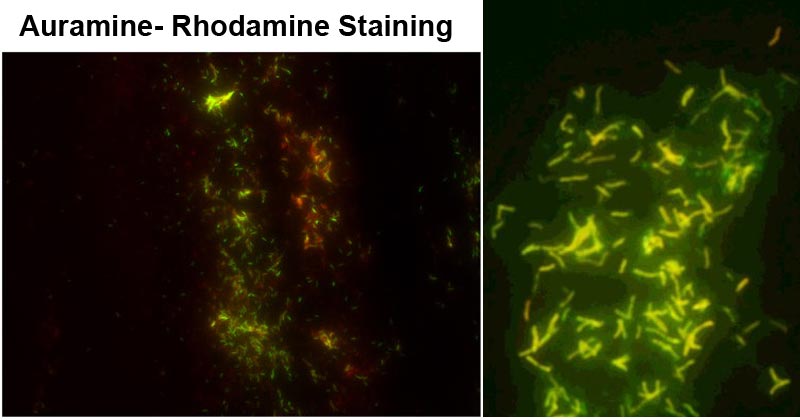
Figure 1: M. tuberculosis stained by fluorescence auramine–rhodamine. Image Source: Front. Microbiol., 03 November 2015 | https://doi.org/10.3389/fmicb.2015.01184. Figure 2: Mycobacteria stained with fluorescent auramine-rhodamine stain. Image Source: https://doi.org/10.1016/j.jctube.2016.05.005
Positive Test- Acid-fast bacilli fluoresce red-orange or fluoresce yellow or reddish-yellow fluorescence against a dark background.
Negative Test- Non-acid-bacilli do not fluoresce, and they appear pale yellow.
Applications of Auramine- Rhodamine Staining
- Being a rapid sensitive test, it has been used to identify and demonstrate the process of Mycobacterium spp is sputum and urine samples.
- A sensitivity test to detect Mycobacterium tuberculosis and Mycobacterium leprae.
Advantages of Auramine- Rhodamine Staining
- It is a rapid staining technique compared to the Ziehl-Neelsen technique
- Its more sensitive than the Zeihl- Neelsen stain
- It doesn’t require heat for fixation of dyes
Limitations of Auramine- Rhodamine Staining
- Confirmation by Culture methods is required since positive or negative staining gives presumptive results.
- Most strains of rapid growers may not appear fluorescent.
- Negative fluorescence should be confirmed with the Zeihl Neelsen stain.
- These dyes (Auramine and Rhodamine) are possibly carcinogenic.
- Acid alcohol and Potassium Permanganate can irritate the skin, eyes, and the respiratory tract.
- Excessive exposure to the counterstain may result in a loss of the brilliance of the fluorescing organism.
- Stains should be observed within 24 hours of staining before the fluorescence fades away.
Reference and Sources
- Smyczek, Petra & Verity, Robert & Kanwal, Surinder & Puttagunta, Lakshmi & Kunimoto, Dennis. (2011). Auramine-Rhodamine Staining in Comparison to Modern Liquid Culture Systems for the Detection of Mycobacterium spp.
- 2% – https://microbeonline.com/auramine-rhodamine-fluorochrome-staining-principle-procedure-results-limitations/
- 2% – https://laboratoryinfo.com/auramine-rhodamine-staining-for-afb-principle-procedure-reporting-and-limitations/
- 1% – https://www.who.int/buruli/information/diagnosis/en/index13.html
- 1% – https://www.termpaperwarehouse.com/essay-on/Acid-Fast-Staining/89799
- 1% – https://www.chemeurope.com/en/encyclopedia/Mycolic_acid.html
- <1% – https://www.sigmaaldrich.com/catalog/product/sial/51362
- <1% – https://www.answers.com/Q/Why_is_acid_alcohol_rather_than_ethyl_alcohol_used_as_a_decolorizing_agent_in_the_acid_fast_staining_experiment
- <1% – https://microbiologyinfo.com/acid-fast-stain-principle-procedure-interpretation-and-examples/

I like ur notes coz thy are clear and brief ,,, easy to understand