What is Mueller Hinton Agar (MHA)?
Mueller and Hinton developed Mueller Hinton Agar (MHA) in 1941 for the isolation of pathogenic Neisseria species. Mueller Hinton agar is a microbiological growth medium that is commonly used for antibiotic susceptibility testing using the Kirby-Bauer disc diffusion method. MHA is recommended for the diffusion of antimicrobial agents impregnated on paper disc through an agar gel as described in CLSI Approved Standard. Zone diameters established for each antimicrobial determining resistant, intermediate, and sensitive results for pathogenic microorganisms are listed in the Clinical and Laboratory Standards Institute (CLSI). Mueller Hinton Agar with 5% sheep blood and Mueller Hinton Agar with Hemoglobin has been recommended for antimicrobial susceptibility testing of Streptococcus pneumoniae and Haemophilus influenza. MHA has been selected by the CLSI for several reasons:
- It demonstrates good batch-to-batch reproducibility for susceptible testing.
- It is low in sulfonamide, trimethoprim and tetracycline inhibitors.
- It supports the growth of most non-fastidious bacterial pathogens.
- Many data and much experience regarding its performance have been recorded.
Why MHA is used for antibiotic susceptibility testing?
- It is a non-selective, non-differential medium. This means that almost all organisms plated on here will grow.
- It contains starch. Starch is known to absorb toxins released from bacteria, so that they cannot interfere with the antibiotics. It also mediates the rate of diffusion of the antibiotics through the agar.
- It is a loose agar. This allows for better diffusion of the antibiotics than most other plates. A better diffusion leads to a truer zone of inhibition.
- MHA shows acceptable batch-to-batch reproducibility for susceptibility testing.
- MHA is low in sulfonamide, trimethoprim, and tetracycline inhibitors (i.e. concentration of inhibitors thymidine and thymine is low in MHA).
- Both the para-aminobenzoic acid (PABA) and thymine/thymidine content in Mueller Hinton Agar are reduced to a minimum, thus markedly reducing the inactivation of sulfonamides and trimethoprim when the media is used for testing the susceptibility of bacterial isolates to these antimicrobics.
Principle of Mueller Hinton Agar (MHA)
Mueller Hinton Agar media contains Beef Extract, Acid Hydrolysate of Casein, Starch, and Agar. Beef Extract and Acid Hydrolysate of Casein provide nitrogen, vitamins, carbon, amino acids, sulfur, and other essential nutrients. Starch acts as a colloid and is added to absorb any toxic metabolites produced. Starch hydrolysis yields dextrose, which serves as a source of energy. Agar is the solidifying agent. The levels of tetracycline and sulfonamide inhibitors, thymidine, thymine, magnesium, and calcium ions, are controlled so as not to interfere with susceptibility testing and to yield good growth. The use of a suitable medium for testing the susceptibility of microorganisms to sulfonamides and trimethoprim is essential. Antagonism to sulfonamide activity is demonstrated by para-aminobenzoic acid (PABA) and its analogs. Reduced activity of trimethoprim, resulting in smaller inhibition zones and inner zonal colonies, is demonstrated on unsuitable Mueller Hinton medium possessing high levels of thymidine. Both the PABA and thymine/thymidine content in Mueller Hinton Agar are reduced to a minimum, thus markedly reducing the inactivation of sulfonamides and trimethoprim when the media is used for testing the susceptibility of bacterial isolates to these antibiotics.
Composition of Mueller Hinton Agar (MHA)
| Ingredients | Gms/Litre |
| Beef extract | 2.0 |
| Acid hydrolysate of casein | 17.5 |
| Starch | 1.5 |
| Agar | 17.0 |
Final pH 7.3 +/- 0.1 at 25ºC.
Preparation of Mueller Hinton Agar (MHA)
- Suspend 38 gm of the medium in 1000ml of distilled water.
- Heat with frequent agitation and boil for one minute to completely dissolve the medium.
- Autoclave at 121°C for 15 minutes. Cool to room temperature.
- Pour cooled Mueller Hinton Agar into sterile petri dishes on a level, horizontal surface to give uniform depth.
- Allow to cool to room temperature.
- Store the plates at 2-8 ºC.
Result on Mueller Hinton Agar (MHA)
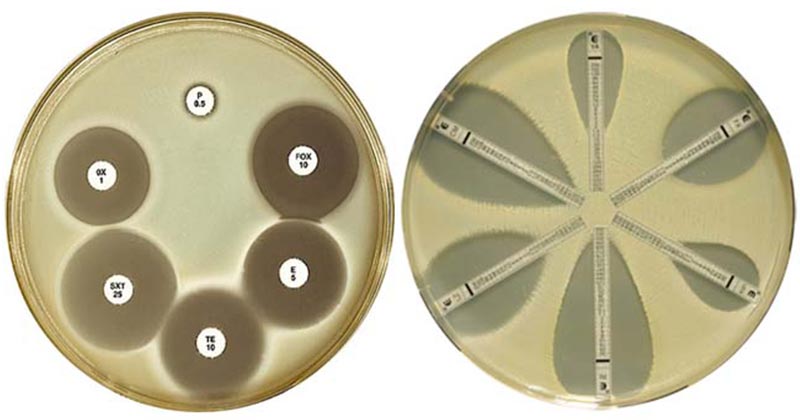
Zone of inhibition is observed around the antibiotics
Uses
- The major use of Mueller Hinton Agar is for antimicrobial susceptibility testing. It has become the standard medium for the Bauer Kirby method and its performance is specified by the NCCLS.
- It can be used to cultivate Neisseria.
- It is specified in FDA Bacteriological Analytical Manual for food testing, and procedures commonly performed on aerobic and facultative anaerobic bacteria.
Limitations
- Numerous factors can affect results: inoculum size, rate of growth, medium formulation and pH. Strict adherence to protocol is required to ensure reliable results.
- Inoculum density may effect the zone size. Heavy inoculum may result in smaller zones or too less inoculum may result in bigger zones.
- Fastidious organisms may not grow on this medium and may require supplementation of blood.
- Fastidious anarobes may not grow on this medium.
- The disk diffusion method should not be used for obligatory anaerobes, slow growing organisms, and capnophiles. This method was standardized for facultative organisms or rapid growing aerobes.
- Drug inactivation may result from the prolonged incubation times required by slow growers.
- Variation in the concentration of divalent cations, primarily calcium and magnesium affects result of aminoglycoside, tetracycline, and colistin test with P. aeruginosa isolates.
References
- Atlas R.M and Snyder J.W. 2014. Handbook of Media for Clinical and Public Health Microbiology. CRC Press. Taylor & Francis Group. 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742. Page no.324-325
- https://en.wikipedia.org/wiki/Mueller-Hinton_agar
- http://himedialabs.com/TD/M173.pdf
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/MuellerHintonMed.htm
- https://microbiologyinfo.com/mueller-hinton-agar-mha-composition-principle-uses-and-preparation/
- https://microbeonline.com/why-mueller-hinton-agar-is-used-in-routine-antibiotic-susceptibility-testing/

Nice information. Thanks. I use MH regularly in Microbiology and the information here is good and compact .
Why is that fastidious organisms may not grow on this medium