Minor pathways of carbohydrate metabolism are alternative, quantitatively smaller oxidative routes of glucose that operate alongside glycolysis and the TCA cycle.
Their primary function is not ATP generation, but the production of specialized compounds such as NADPH, pentoses, and glucuronic acid.
Key examples are the pentose phosphate pathway (HMP shunt), uronic acid (glucuronic acid) pathway, polyol (sorbitol) pathway, and the hexosamine biosynthetic pathway.

Pentose Phosphate Pathway (HMP Shunt): Generating NADPH and Ribose
- The pentose phosphate pathway is an alternative pathway to glycolysis and is also known as the Hexose Monophosphate Shunt (HMP shunt), Phosphogluconate Pathway, and Shunt Pathway.
- This pathway is divided into an oxidative (irreversible) phase, which produces NADPH, and a non-oxidative (reversible) phase, which rearranges sugar phosphates to generate ribose-5-phosphate or return intermediates to glycolysis.
- This pathway is active in the cytosol of many cells, e.g., liver, adipose tissues, adrenal cortex, ovaries, testis, RBCs, and retina.
- PPP starts with glucose 6-phosphate.
- 10% of glucose per day enters this pathway.
- No ATP is directly utilized or produced.
Functions of NADPH (from PPP/HMP shunt)
- Produced in the oxidative phase of the pentose phosphate pathway when glucose-6-phosphate and 6-phosphogluconate are oxidized
- Serves as a major reducing equivalent for anabolic pathways, including the synthesis of fatty acids, cholesterol, and steroid hormones in the liver, adipose tissue, adrenal cortex, and mammary gland.
- Fuels glutathione reductase to convert oxidized glutathione (GSSG) back to reduced glutathione (GSH), which detoxifies hydrogen peroxide and other reactive oxygen species via glutathione peroxidase.
- Maintains cellular redox balance, protecting membranes, proteins, and DNA from oxidative damage, especially in high-oxygen or high-ROS environments.
- In erythrocytes, which lack mitochondria, the PPP is the main source of NADPH; this NADPH keeps hemoglobin in the reduced functional state and preserves red cell membrane integrity, preventing hemolysis.
- Supports the respiratory burst in phagocytes by providing electrons for NADPH oxidase to generate superoxide, hydrogen peroxide, and hypochlorous acid used to kill ingested microbes.
Functions of ribose-5-phosphate
- Generated mainly in the non-oxidative phase when ribulose-5-phosphate is converted to ribose-5-phosphate by isomerase- and transketolase-dependent reactions.
- Provides ribose for the synthesis of DNA and RNA precursors, serving as the sugar backbone for nucleotides.
- It helps produce the adenine and guanine nucleotides (ATP, GTP, UTP, and CTP) needed for metabolism, energy transfer, and signal transduction.
- It connects the PPP to several regulatory pathways by supplying ribose for coenzymes like NAD⁺, FAD, and NADP as well as second messengers like cAMP and cGMP.
- It serves as the primary physiological source of ribose because tissues do not have a particular kinase that can phosphorylate free dietary ribose to R5P; as a result, extra dietary ribose is eliminated rather than utilized.
- This is particularly crucial in rapidly dividing cells (such as bone marrow, skin, mucosa, and tumors), where high rates of nucleotide synthesis are necessary to produce RNA and DNA replication.
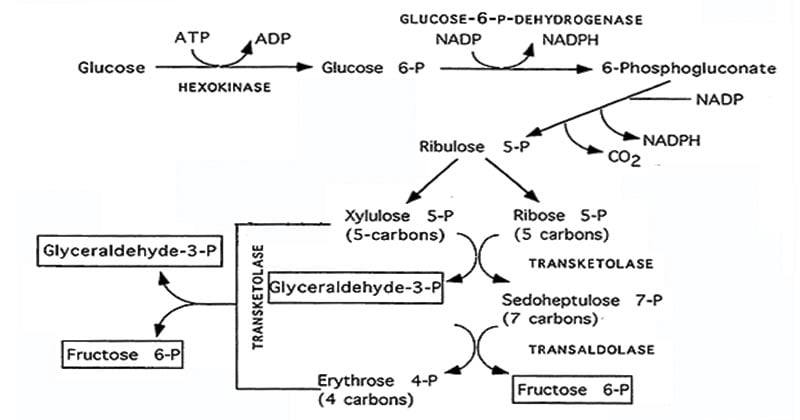
Oxidative vs. Non-Oxidative Phases of the HMP Shunt
Reactions of the pentose phosphate pathway occur in the cytosol in two phases.
The oxidative phase
- The first, irreversible stage of the pentose phosphate pathway is called the oxidative phase, and its primary function is to produce NADPH while converting glucose 6-phosphate into ribulose 5-phosphate and CO2.
- Step 1: Glucose-6-phosphate is oxidized by glucose-6-phosphate dehydrogenase (G6PD) to form 6-phosphogluconolactone.
- During this oxidation, electrons are transferred from glucose-6-phosphate to NADP⁺, producing the first molecule of NADPH.
- Step 2: 6-phosphogluconolactone is then hydrolyzed by 6-phosphogluconolactonase, which opens the ring to give the linear sugar acid 6-phosphogluconate, preparing it for the next oxidative decarboxylation step.
- Step 3: 6-phosphogluconate dehydrogenase converts 6-phosphogluconate to ribulose-5-phosphate (Ru5P) while generating the second molecule of NADPH. So each G6P yields 2 NADPH, 1 ribulose-5-phosphate, and 1 CO₂ in this phase.
- These oxidative reactions take place in the cytoplasm, and G6PD serves as the main regulatory point.
- Its activity rises when NADP⁺ and oxidative stress are high and falls when NADPH is abundant, aligning NADPH production with the cell’s demand for reducing power.
Non-Oxidative Phases
- The non-oxidative phase consists of a bunch of reversible, carbon-carbon rearrangements.
- Its primary purpose is to generate pentose phosphates, chiefly ribose-5-phosphate, which is important for nucleotide synthesis.
- This phase begins when ribulose-5-phosphate (Ru5P), produced in the oxidative phase, is converted to ribose-5-phosphate (R5P) and xylulose-5-phosphate by specific isomerase and epimerase enzymes.
- R5P is then available as a key precursor for nucleotide synthesis, providing the ribose moiety for DNA, RNA, and nucleotide triphosphates such as ATP and GTP that are essential for energy transfer and signaling.
- When there is more R5P than the cell needs for nucleotides, transketolase (TPP-dependent) and transaldolase transfer 2-carbon and 3-carbon units between pentose phosphates (like R5P and xylulose-5-P) and larger sugars (such as sedoheptulose-7-P).
- Through these carbon-transfer reactions, pentose sugars are rearranged into fructose-6-phosphate and glyceraldehyde-3-phosphate, which can re-enter glycolysis or gluconeogenesis, linking the pentose phosphate pathway back to central carbohydrate metabolism.
- All the reactions are reversible, which means fructose-6-phosphate and glyceraldehyde-3-phosphate from glycolysis can be converted back into ribose-5-phosphate when the cell needs more nucleotides but does not need more NADPH.
- This means that fructose 6 phosphate and glyceraldehyde 3 phosphate from glycolysis can be converted back into ribose 5 phosphate when the cell needs more nucleotides but does not need more NADPH.
The Uronic Acid Pathway: Synthesis of Glucuronic Acid
- The uronic acid pathway is an alternative pathway for glucose metabolism that produces glucuronic acid.
- This is important for conjugating bilirubin, steroids, and drugs to make them more water-soluble and excretable.
- It occurs mainly in the cytosol of the liver (and to a lesser extent kidney), so it is considered a minor pathway of glucose oxidation alongside the PPP/HMP shunt.
- This pathway also produces Ascorbic Acid in certain animals.
- The unutilized Glucuronate produced in this pathway is converted to Xylulose 5 5-phosphate, which is further metabolized through PPP.
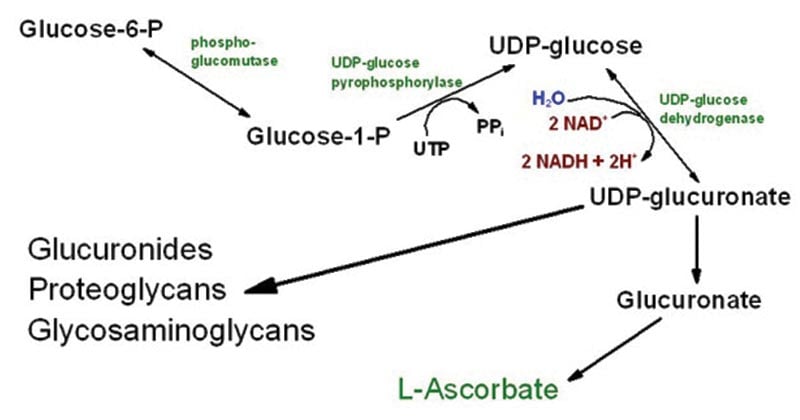
Steps of the uronic acid pathway
Formation of UDP-glucuronate
- Glucose is phosphorylated by hexokinase/glucokinase to glucose-6-phosphate
- Glucose-6-phosphate is first converted to glucose-1-phosphate, then to UDP-glucose by UDP-glucose pyrophosphorylase.
- UDP‑glucose dehydrogenase oxidizes UDP‑glucose to UDP‑glucuronate, the active form of glucuronic acid.
UDP‑glucuronate → L‑gulonate
- UDP‑glucuronate is hydrolyzed to D‑glucuronate, releasing UDP.
- D‑glucuronate is then reduced in an NADPH‑dependent reaction to form L‑gulonate.
L‑gulonate and vitamin C (ascorbic acid)
- In many animals, L‑gulonate is the precursor for ascorbic acid (vitamin C).
- The key enzyme L‑gulonolactone oxidase converts gulonolactone to ascorbic acid.
Oxidation of L-gulonate
- L-gulonate is oxidized to 3-ketogulonate and then converted to L-xylulose.
- L-xylulose is further changed into D-xylulose via xylitol through NADPH- and NAD⁺-dependent reactions.
- After phosphorylation, D-xylulose enters the pentose phosphate pathway for further metabolism.
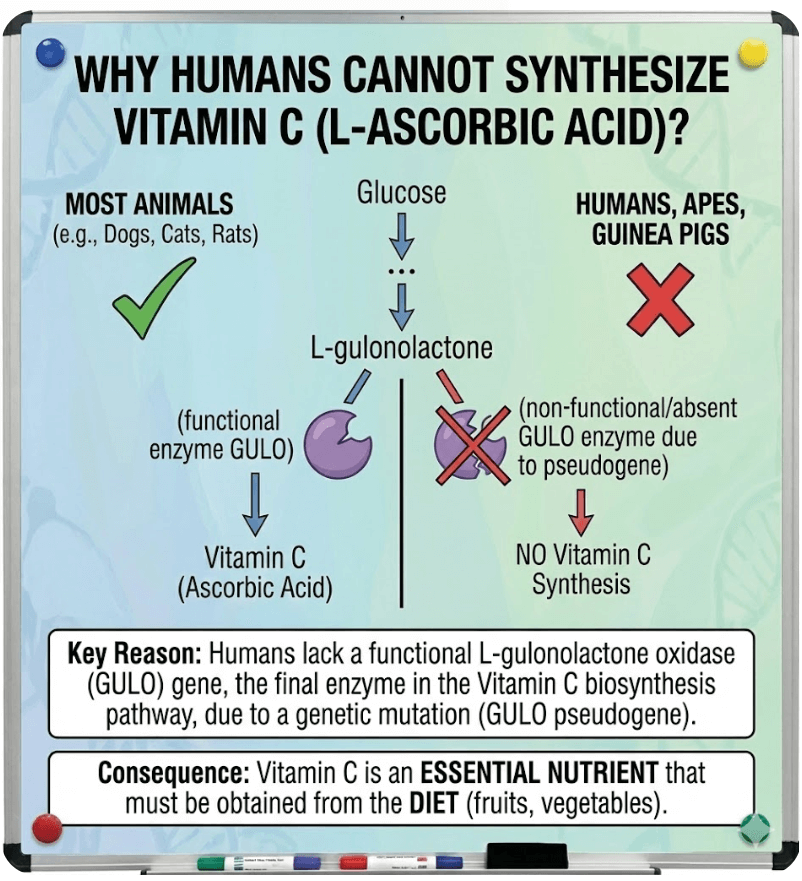
Biological Significance: Why Humans Cannot Synthesize Vitamin C
- Most animals produce vitamin C from glucose-6-phosphate through a process that results in the conversion of L-gulono-1,4-lactone to L-ascorbic acid by L-gulono-1,4-lactone oxidase (GULO).
- GULO is essential for forming the redox-active C2–C3 double bond of L ascorbic acid; it belongs to the aldonolactone oxidoreductase family of flavoenzymes.
- In primates, including humans, this GULO encoding gene has undergone extensive mutations, so the enzyme is no longer functional, and the biosynthetic pathway cannot produce L-ascorbic acid.
- GULO is essential for forming the redox-active C2–C3 double bond of L-ascorbic acid; it belongs to the aldonolactone oxidoreductase family of flavoenzymes.
- In primates, including humans, this GULO encoding gene has undergone extensive mutations, so the enzyme is no longer functional, and the biosynthetic pathway cannot produce L-ascorbic acid.
The Polyol Pathway (Sorbitol Pathway): Glucose to Fructose Conversion
The polyol pathway is a metabolic pathway where a part of excess glucose is metabolized to sorbitol, which is then converted to fructose.
Step 1 – Glucose → Sorbitol (rate‑limiting step)
- Excess glucose is converted into sorbitol by the enzyme aldose reductase, using NADPH as a reducing agent.
- Normally, this step is slow, but during hyperglycemia, more glucose enters insulin-independent tissues (lens, nerves, kidney), making this pathway highly active.
Step 2 – Sorbitol → Fructose
- Sorbitol is converted into fructose by sorbitol dehydrogenase, using NAD⁺ and producing NADH.
- This step helps process sorbitol, but in tissues with low sorbitol dehydrogenase, sorbitol can accumulate and cause osmotic stress.
Physiological Roles of the Polyol Pathway
- The polyol pathway diverts excess glucose in insulin-independent tissues.
- It produces fructose as an alternative energy source, especially for sperm cells.
- Sorbitol helps protect cells by maintaining osmotic balance.
- NADPH use in this pathway affects cellular antioxidant defense.
- It supports metabolic adaptation during cellular stress.
Metabolism of Amino Sugars: The Hexosamine Biosynthetic Pathway
- When a hydroxyl group of sugar is replaced by an amino group, the resultant compound is an amino sugar.
- It is estimated that about 20% of the glucose is utilized for the synthesis of amino sugars, which mostly occurs in the connective tissue.
- The hexosamine biosynthesis pathway is a specialized branch of glycolysis, the primary glucose breakdown pathway. It diverts a small, controlled amount of glucose-6-phosphate (typically 2-5% of total glucose flux) to produce a unique sugar derivative.
- This pathway integrates multiple nutrient inputs, including glucose, amino acids like glutamine, fatty acids, and ATP.
Steps of the Hexosamine Biosynthetic Pathway
From glycolysis to entry: A small portion of glucose is converted to glucose‑6‑phosphate and then to fructose‑6‑phosphate, which is siphoned off from the main glycolytic pathway into the hexosamine route.
Committed step (GFAT): Fructose‑6‑phosphate combines with glutamine in a reaction catalyzed by GFAT1/2, producing glucosamine‑6‑phosphate; this is the rate‑limiting, commitment step of the hexosamine biosynthetic pathway.
Acetylation (GNPNAT1): Glucosamine‑6‑phosphate is acetylated using acetyl‑CoA by GNPNAT1 to form N‑acetylglucosamine‑6‑phosphate (GlcNAc‑6‑P), linking the pathway to cellular acetyl‑CoA and fat/energy status.
Rearrangement (PGM3): GlcNAc‑6‑phosphate is isomerized by PGM3 to N‑acetylglucosamine‑1‑phosphate (GlcNAc‑1‑P), preparing it for activation as a sugar nucleotide.
Activation to UDP‑GlcNAc (UAP1): UAP1 then couples GlcNAc‑1‑phosphate with UTP to generate UDP‑GlcNAc, the activated amino‑sugar donor used for N‑glycosylation, O‑GlcNAcylation, and synthesis of many glycoconjugates throughout the cell.
Clinical Significance: G6PD Deficiency and Hemolytic Anemia
- G6PD deficiency is an X-linked inherited enzymatic disorder that impairs the pentose phosphate pathway, reducing NADPH production in red blood cells.
- NADPH is essential for maintaining reduced glutathione, which protects erythrocytes from oxidative damage; deficiency increases vulnerability to oxidative stress
- Red blood cells lack nuclei and mitochondria, making them entirely dependent on G6PD for antioxidant defense, predisposing them to hemolysis.
- Exposure to oxidative triggers such as infections, fava beans, and certain drugs (e.g., antimalarials, sulfonamides) can precipitate acute hemolytic anemia.
- Oxidative stress leads to hemoglobin denaturation and Heinz body formation, resulting in membrane damage and premature red cell destruction.
- Clinically, hemolytic episodes present with pallor, jaundice, fatigue, dark urine, and anemia
- In neonates, G6PD deficiency is a significant cause of severe hyperbilirubinemia and may lead to kernicterus if not promptly managed.
Diabetic Complications: The Role of Sorbitol Accumulation
- Persistent hyperglycemia in uncontrolled diabetes mellitus allows extra glucose to enter insulin-independent tissues like the kidneys, lens, retina, and peripheral nerves.
- As these tissues have sufficient NADPH and high aldose reductase activity, glucose is quickly converted to sorbitol through the polyol (sorbitol) pathway.
- These cells have little or no sorbitol dehydrogenase activity, which prevents sorbitol from being effectively converted to fructose.
- Because sorbitol is poorly permeable through cell membranes, it builds up inside the cells that produce it.
- Since sorbitol is hydrophilic, it accumulates and causes osmotic stress, causing structural damage and cellular swelling.
- Major diabetic complications like cataracts, peripheral neuropathy, nephropathy, and retinopathy get worse due to this osmotic and oxidative damage.
- Sorbitol accumulation is regarded as a common pathogenic factor since it is the source of several diabetic complications.
Conclusion
- Carbohydrate minor metabolic pathways, which provide specialized products like NADPH, ribose-5-phosphate, glucuronic acid, and amino sugars rather than ATP production, offer crucial substitutes for glycolysis and the TCA cycle.
- HMP Shunt, or Pentose Phosphate Pathway: The non-oxidative phase creates ribose-5-P for nucleotides and recycles intermediates to glycolysis, while the oxidative phase produces NADPH for redox balance and biosynthesis.
- Humans lack functional L-gulonolactone oxidase, which prevents the synthesis of vitamin C. The uronic acid pathway produces glucuronic acid for detoxification.
- The polyol (sorbitol) pathway contributes to osmotic stress in diabetic complications like neuropathy and retinopathy by converting excess glucose to sorbitol and fructose.
- The hexosamine biosynthesis pathway connects glucose to nutrient sensing by altering fructose-6-P to UDP-GlcNAc for glycosylation and glycoconjugates.
- These pathways underlie conditions such as sorbitol accumulation, exacerbating damage from hyperglycemia and G6PD deficiency, causing hemolytic anemia due to impaired NADPH production.
References
- Boverio, A., Jamil, N., Mannucci, B., Mascotti, M. L., Fraaije, M. W., & Mattevi, A. (2024). Structure, mechanism, and evolution of the last step in vitamin C biosynthesis. Nature communications, 15(1), 4158. https://doi.org/10.1038/s41467-024-48410-1
- Chandel N. S. (2021). Carbohydrate Metabolism. Cold Spring Harbor perspectives in biology, 13(1), a040568. https://doi.org/10.1101/cshperspect.a040568
- Gugliucci, A. (2017). Dietary sugars and endogenous formation of advanced glycation endproducts: Emerging mechanisms of disease. Nutrients, 9(4), 385. https://doi.org/10.3390/nu9040385
- Mak, G. K., & Shah, M. (2025). Glucose-6-phosphate dehydrogenase deficiency. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK470315/
- Paneque AL et al. The Hexosamine Biosynthesis Pathway: Regulation and Function. Int J Mol Sci. 2023. Open access: https://pmc.ncbi.nlm.nih.gov/articles/PMC10138107/
- Satyanarayana, U., & Chakrapani, U. (2017). Biochemistry (5th ed.). Elsevier.
- Srikanth, K. K., & Orrick, J. A. (2022). Biochemistry, polyol or sorbitol pathways. StatPearls. StatPearls Publishing. Retrieved December 23, 2025, from https://www.ncbi.nlm.nih.gov/books/NBK576381/
