The major histocompatibility complex can be defined as a tightly linked cluster of genes whose products play an important role in intercellular recognition and in discrimination between self and non-self. The term ‘histo’ stands for tissue and ‘compatibility’ refers to ‘getting along or agreeable’. On the other hand, the term ‘complex’ refers to the ‘genes that are localized to a large genetic region containing multiple loci’. These genes code for antigens which involve the determination of the compatibility of the transplanted tissue. The compatible tissues will be accepted by the immune system while the histo-incompatible ones are rejected. The rejection of foreign tissue leads to an immune response to cell surface molecules. The concept was first identified by Peter Gorer and George Snell. The main function of MHC molecules is to bring antigen to the cell surface for recognition by T cells. In humans, the genes coding for MHC molecules is found in the short arm of chromosome 6.
Interesting Science Videos
Major Histocompatibility Complex (MHC) Molecules Characteristics
- The Major Histocompatibility complex is a genetic locus that encodes the glycoprotein molecules (transplantation antigens) which are responsible for tissue rejection of grafts between genetically unidentical individuals.
- It is also the molecule that binds the peptide antigens processed by Antigen-presenting Cells and presents them to T-cells, hence they are responsible for antigen recognition by the T-cell receptors.
- Unlike the B-cell receptors that directly interact with the antigens, the T-cell receptors have an intertwined relationship with the MHC molecule, in that T-cell receptors can only receive and bind processed antigens in form of peptides that are bound to the MHC molecule, and therefore, T-cell receptors are specific for MHC molecules.
- In humans, the Major Histocompatibility complex is known as Human Leukocyte Antigen (HLA). There are three common MHC molecules i.e class I, class II, and class III MHC proteins.
- The genes of the MHC exhibit genetic variability; and the MHC has several genes for each class hence it is polygenic.
- The MHC is also polymorphic, meaning a large number of alleles exist in the population for each of the genes.
- Therefore, a large number of alleles exist in the population for each of the genes. Each individual inherits a restricted set of alleles from his or her parent. Sets of MHC genes tend to be inherited as a block or haplotype. There are relatively infrequent cross-over events at this locus.
- The structure of the MHC class I have two domains that are distant from each other, made up of two parallel α helices on top of a platform that is created by a β-pleated sheet. The general structure looks like a cleft whose sides are formed by the α helices and the floor is β-sheet.
- Generally, the MHC molecules have a broad specificity for peptide antigens and many different peptides can be presented by any given MHC allele binding a single peptide at a time.
- The α helices forming the binding clefts are the site of the amino acid residues that are polymorphic (varying allelic forms) in MHC proteins, meaning that different alleles can bind and present different peptide antigens. For all these reasons, MHC polymorphism has a major effect on antigen recognition.
- The function of T-cells on interaction with the MHC molecules reveals that the peptide antigens associated with class I MHC molecules are recognized by CD8+ cytotoxic T-lymphocytes (Tc cells) and MHC class-II associated with peptide antigens that are recognized by CD4+ Helper T-cells (Th cells).
- The MHC in humans is known as human leukocyte antigens (HLA) complex.
HLA complex
In humans, the HLA complex of genes is located on short arm of chromosome 6 containing several genes that are critical to immune function. The HLA complex of genes is classified into three classes as follows:
- Class I: HLA-A, HLA-B, and HLA-C.
- Class II: HLA-DR, HLA-DQ, and HLA-DP. All of these are present within HLA-D region of HLA complex.
- Class III: Complement loci that encode for C2, C4, and factor B of complement system and TNFs alpha and beta.
Gene Products of HLA complex
- Class I MHC genes encode glycoproteins expressed on the surface of nearly all nucleated cells; the major function of the class I gene products is presentation of endogenous peptide antigens to CD8+ T cells.
- Class II MHC genes encode glycoproteins expressed predominantly on APCs (macrophages, dendritic cells, and B cells), where they primarily present exogenous antigenic peptides to CD4+ T cells.
- Class III MHC genes encode several different proteins, some with immune functions, including components of the complement system and molecules involved in inflammation.
Major Histocompatibility Complex (MHC) Types
In humans, the MHC molecules are divided into three types, Class I, Class II and Class III. Class I MHC molecules are coded from three different locations called A, B and C and these molecules are expressed in all nucleated cells. Class II MHC genes are located in the D region and there are several loci such as DR, DQ and DP and these molecules are expressed only in antigen-presenting cells. Class III MHC genes are coded in the region between Class I and Class II genes. Class III MHC genes codes for cytokines and complement proteins which play an important role during the immune response.
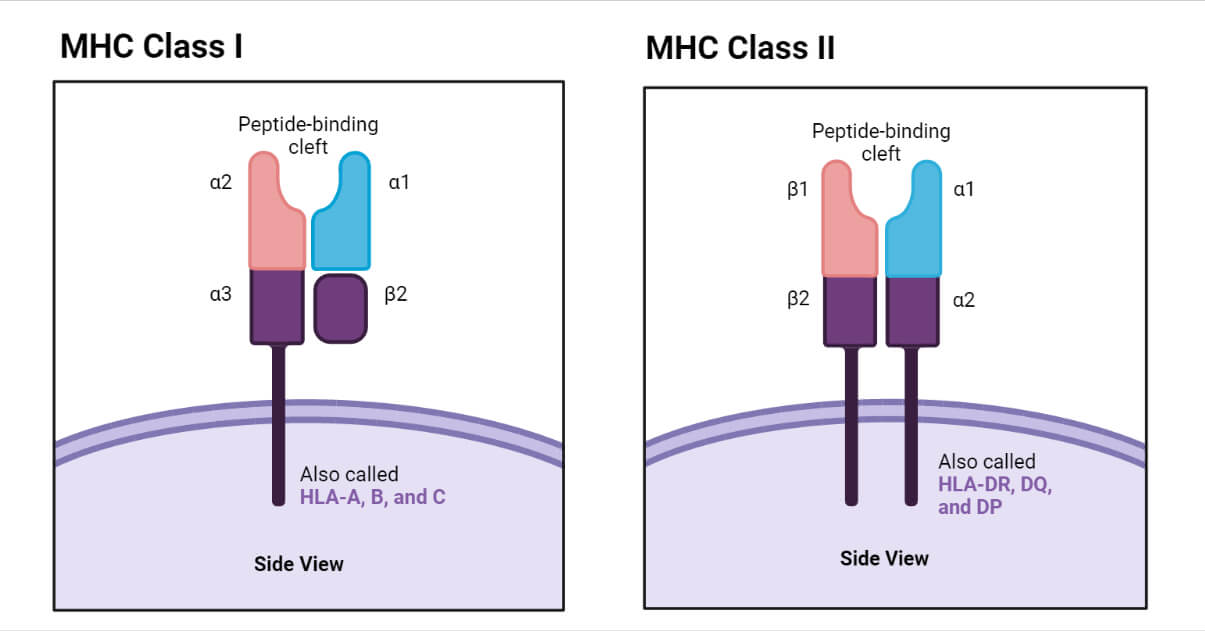
Class I MHC Molecule
- The structure of Class I MHC molecule consists of two polypeptide chains α and β. These two chains are connected together by non-covalent bonds. The α chain is characterized as an internal membrane glycoprotein with a molecular weight of 45000 Da (in humans). Β chain, on the other hand, is an extracellular microglobulin with a molecular mass of 12kDa.
- The α chain is made up of approximately 350 amino acids and also divided into three globular domains α1, α2 and α3. Each of these domains contains roughly 90 amino acids. The N terminal of α chain is the place of α1 domain, while α2 and α3 are present after α1 The α2 domain is characterized by the formation of a loop of 63 amino acids; the loop is formed due to intrachain disulfide bond. α3 also contains a disulfide bond enclosing 86 amino acids. The α1 and α2 domains interact to form peptide-binding units of class I MHC molecule.
- Moreover, α chain also consists of a stretch of 26 hydrophobic amino acids which holds the α chain on the plasma membrane. This transmembrane segment is present as a form of α helix at the hydrophobic region of the plasma membrane. An intracellular domain or the carboxyl-terminal of α chain is located inside the cell and it contains around 30-40 amino acids.
- In humans the β chain is non-polymorphic and it is dimorphic in mice. α3 and β chain are structurally similar to the immunoglobulin C domain and also characterized as a disulfide loop. A peptide binding platform is formed by β plated sheets of α1 and α2
- Tcyt Cell (cytotoxic T cell) has specificity towards cells containing peptides associated with Class I MHC due to the presence of CD8 antigen on the surface of Tcyt Cell. CD8 antigen has an affinity towards the α3 domain of Class I MHC molecules.
Class II MHC Molecule
- Class II MHC molecules are heterodimers and characterized by two non-covalently connected polypeptide chains. The chains are termed a heavy chain (α, 30kDa) and light chain (β, 26kDa).
- Similar to class I MHC molecules, class II MHC molecules are also characterized by an extracellular amino-terminal domain, a transmembrane domain, and an intracellular carboxy-terminal tail.
- The class II MHC molecules are expressed on the surface of the antigen-presenting cells such as B cells, dendritic cells, and macrophages.
- The α chain is divided into two domains α1 and α2, while the β chain is also divided into two groups β1 and β2. The β2 domain is responsible for the binding of T cell co-receptor CD4. The α1 and β1 domains, on the other hand, are involved in the formation of the antigen-binding sites. Peptides containing 13-20 amino acids can bind at the antigen-binding site of class II MHC.
- The presence of disulfide bonds in α2, β1, and β2 domains is also an important structural feature of the class II MHC molecules.
Class III MHC Molecule
- There are several serum proteases which involve in the complement system that come under the group of class III MHC molecules.
- Class III MHC molecules do not have any involvement in antigen presentation.
- The complement components such as asC2, C4A, and C4B, and factor B are the most important compounds involved as class III MHC molecules. Apart from these tumor necrosis factors α and β and some heat shock proteins also come under this category.
Distribution of MHC
Essentially, all nucleated cells carry classical class I molecules. These are abundantly expressed on lymphoid cells, less so on the liver, lung, and kidney, and only sparsely on the brain and skeletal muscle. In humans, the surface of the villous trophoblast lacks HLA-A and -B and bears HLA G, which does not appear on any other body cell. Class II molecules are also restricted in their expression, being present only on antigen-presenting cells (APCs) such as B-cells, dendritic cells, and macrophages and on the thymic epithelium. When activated by agents such as interferon g, capillary endothelia, and many epithelial cells in tissues other than the thymus, they can develop surface class II and increase expression of class I.
They function as cell surface markers enabling infected cells to signal cytotoxic and helper T-cells.
Importance of MHC
- Antibody molecules interact with antigen directly but the T-Cell Receptor (TCR) only recognizes antigen presented by MHC molecules on another cell, the Antigen Presenting Cell. The TCR is specific for the antigen, but the antigen must be presented on a self-MHC molecule.
- The TCR is also specific to the MHC molecule. If the antigen is presented by another allelic form of the MHC molecule in vitro (usually in an experimental situation), there is no recognition by the TCR. This phenomenon is known as MHC restriction.
Peptide antigens associated with class I MHC molecules are recognized by CD8+ cytotoxic T lymphocytes, whereas class II-associated peptide antigens are recognized by CD4+ helper T cells.
Antigen Presentation and Processing
The T cells can recognize the foreign antigen when the antigen is attached to the MHC molecules as an MHC peptide complex. The formation of the MHC-peptide complex requires the degradation of protein antigen in several steps. The degradation process is known as antigen processing. These degraded proteins are then attached to the MHC molecules inside the cell and then the MHC molecules are transported to the membrane to present the antigen with the T cell.
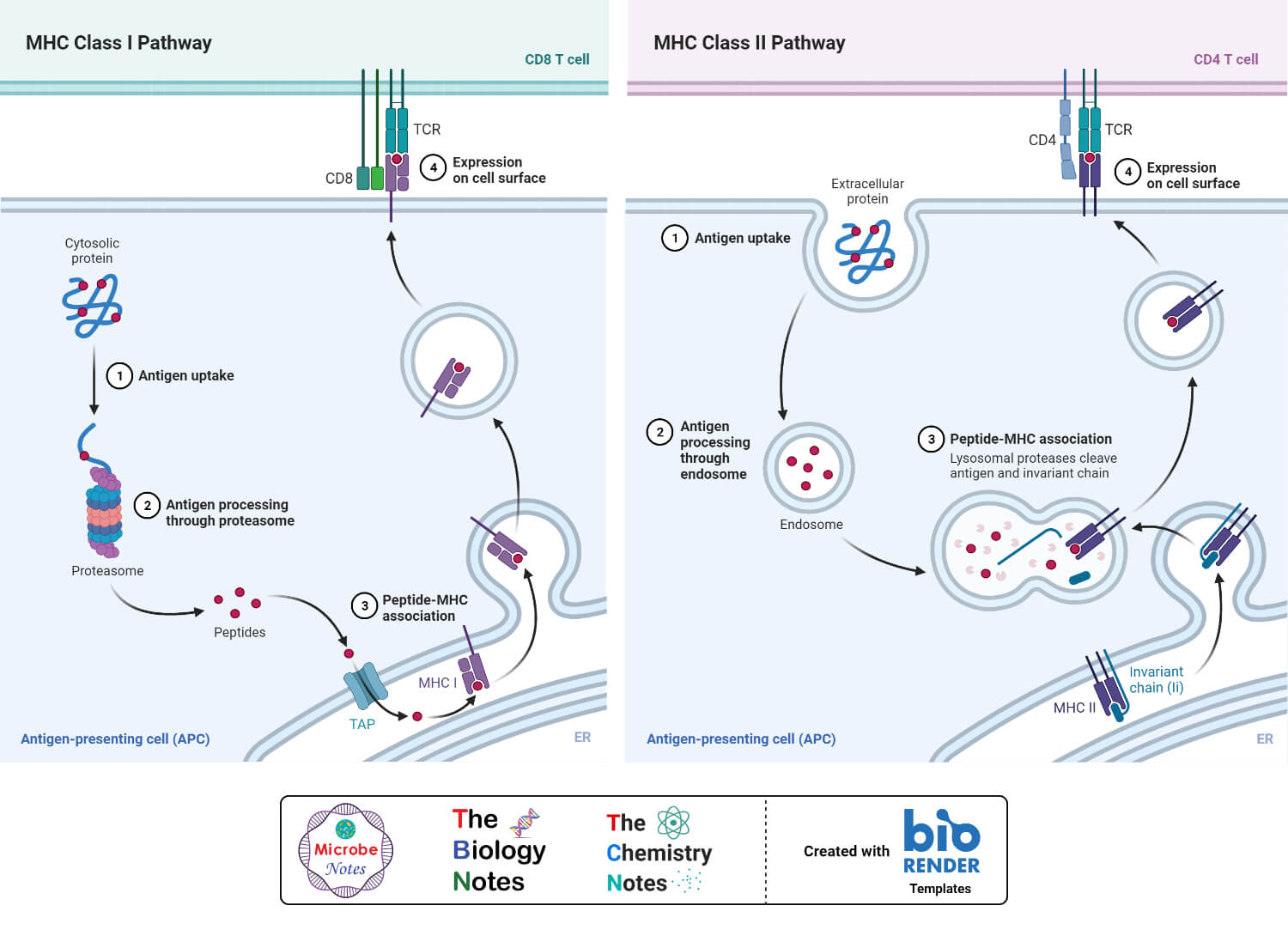
Antigen Presentation Pathway: Class I MHC molecules (Cytosolic pathway)
- Class I MHC molecules involve in presenting intracellular or endogenous pathogens or antigens. Intracellular pathogens refer to those organisms which live and replicates inside the host cell. An example of this type of pathogen is a virus.
- Under normal condition the MHC class I molecules forms a complex with the self-peptides or self-antigens. While, in case of any viral infection, the MHC class I molecules present the peptide derived from the virus which is then further recognized by T cells.
- Cell components such as a nucleus, endoplasmic reticulum and Golgi apparatus play an important role in antigen processing and presentation.
- When a virus infected a normal cell, the viral DNA moves inside the cell and produce viral proteins with the help of the host cell mechanisms. The viral proteins are synthesized in the cytosol.
- The cytoplasm also contains a cylindrical protein complex called the proteasome. The main function of the proteosome is to degrade the unwanted or damaged protein into smaller peptides. At the time of viral infection, the viral proteins interacted with the proteosomes present in the cytoplasm. The processing took place in the cytosol and as a result, the proteins are degraded into smaller peptides (8-15 amino acid long).
- In the next step, these fragmented peptides are transported into the endoplasmic reticulum. The transport took place due to a peptide delivery system called the transporter associated with antigen processing (TAP). TAP is made up of two domains or subunits called TAP 1 and TAP 2.
- Inside the endoplasmic reticulum the α and β chains of MHC class I molecules are synthesized and by the help of a group of chaperone proteins, the MHC class I molecule is formed and moves towards the TAP. As a result, the peptides bind at the peptide-binding site of the class I MHC molecule inside the endoplasmic reticulum and forms the MHC class I-peptide complex.
- In the next step, the MHC class I- peptide complex moves to the surface of the Golgi apparatus and by the help of secretory vesicle, it moves towards the surface of the plasma membrane.
- Once the MHC class I-peptide complex reaches the cell surface, the T cell receptors recognize the antigen peptide complex. Moreover, the co-receptor CD8 of the T cell attaches with the α3 domain of the MHC class I molecule. Hence, the antigen is presented to the T cell.
Antigen Presentation Pathway: Class II MHC molecules (Endocytic Pathway)
- MHC class II molecules are responsible for presenting exogenous or extracellular pathogen or antigen. The extracellular pathogen refers to the organisms which can grow and reproduce outside of the host cell. Bacteria, exotoxins, parasites are examples of extracellular antigens. These antigens are taken up by the cell by endocytosis or phagocytosis.
- Only the antigen-presenting cells involved in antigen processing and presentation by MHC class II molecules. These cells include B cells, macrophages, and dendritic cells. The pathway took place only after the engulfment of the antigen by the antigen-presenting cells.
- Inside the cell, the antigen carries a covering called an endosome. The endosome is fused with the lysosome present in the cytoplasm and forms endolysosomes. As a result, the foreign protein is degraded by the proteolytic enzyme present inside the lysosome and small peptides are formed.
- The class II MHC molecules are synthesized and formed in the endoplasmic reticulum. The α and β chain of the molecule is also associated with the invariant chain. This association helps to restrict the binding of self-antigen with the class II MHC molecule. The invariant chain- MHC complex is then transported from the endoplasmic reticulum to the Golgi apparatus and from the Golgi apparatus to another vesicle. Inside the vesicle, the invariant chain is digested and only a small fragment (Class II-associated invariant chain polypeptide: CLIP) is attached with the molecule.
- In the next step, the vesicle containing the MHC class II molecule is then fused with the vesicle containing fragmented peptides. The fragmented peptide is then bound with the MHC class II molecule by displacing the CLIP. This newly formed MHC class II-peptide complex is then transported to the surface of the cell.
- Once at the cell surface, the antigen is presented to the T cells. The T cell recognizes the peptide bound with the MHC class II molecule by the help of the T cell receptor and the CD4 co-receptor binds with the β2 domain of the class II MHC molecule.
References
- Fahim Halim Khan, The Elements of Immunology, Pearson Education.
- Thomas J Kindt, Barbara A Osborne, Richard Goldsby, Kuby Immunology, 6th Edition.
Sources
- 1% – https://www.sciencedirect.com/topics/neuroscience/mhc-class-ii
- 1% – https://www.ncbi.nlm.nih.gov/pubmed/138383
- 1% – https://www.immunology.org/public-information/bitesized-immunology/systems-and-processes/antigen-processing-and-presentation
- 1% – https://quizlet.com/58619813/lecture-16-flash-cards/
- 1% – https://quizlet.com/187935408/chapters-34-nature-of-antigensantibody-structure-and-function-flash-cards/
- 1% – https://quizlet.com/181279410/antigen-presenting-cells-and-mhc-flash-cards/
- 1% – https://en.wikipedia.org/wiki/Transporter_associated_with_antigen_processing
- 1% – https://en.wikipedia.org/wiki/Antigen_processing
- <1% – https://www.youtube.com/watch?v=Fcxc8Gv7NiU
- <1% – https://www.researchgate.net/publication/20904788_Intracellular_transport_of_class_II_MHC_molecules_directed_by_invariant_chain
- <1% – https://www.researchgate.net/publication/13782996_Exit_of_Major_Histocompatibility_Complex_Class_II-Invariant_Chain_p35_Complexes_from_the_Endoplasmic_Reticulum_is_Modulated_by_Phosphorylation
- <1% – https://www.researchgate.net/publication/11672943_Functional_Heavy-chain_Antibodies_in_Camelidae
- <1% – https://www.ncbi.nlm.nih.gov/gene/925
- <1% – https://www.iariajournals.org/life_sciences/lifsci_v5_n34_2013_paged.pdf
- <1% – https://www.britannica.com/science/major-histocompatibility-complex
- <1% – https://quizlet.com/77514652/viruses-flash-cards/
- <1% – https://quizlet.com/73155913/chapter-8-flash-cards/
- <1% – https://quizlet.com/4870793/immunology-final-flash-cards/
- <1% – https://quizlet.com/25646182/janeway-ch-6-flash-cards/
- <1% – https://quizlet.com/2165953/mhc-and-antigen-presentation-flash-cards/
- <1% – https://quizlet.com/101556355/chapter-8-immunology-flash-cards/
- <1% – https://onlinelibrary.wiley.com/doi/10.1111/aos.13753
- <1% – https://en.wikipedia.org/wiki/MHC_II
- <1% – https://en.wikipedia.org/wiki/MHC_class_I
- <1% – https://en.wikipedia.org/wiki/CLIP_(protein)
- <1% – https://courses.lumenlearning.com/boundless-ap/chapter/antigens/

I want to join you as a blogger’s